| Locomotion in Insects |
Insects have been described as 'ideal miniature robots'. Indeed, the way they move is extremely efficient
and is being used as a basis by robotocists to develop locomotion in their own machines!
The locomotive power for insects comes from the thorax and its appendage (legs and wings). The anatomy
of the thorax is illustrated above.
Insect locomotion is characterised by two main advancements: the jointed limbs characteristic of arthropods
that allowed the arthropods to colonise land and flight. The jointed limb of arthropods (arthropod meaning
'jointed leg') evolved in marine forms, such as crustaceans that are often amphibious, for example many
shore crabs. The tough exoskeleton supports the weight of the animal and also has internal projections to
which the muscles attach. If you have ever eaten a crab claw, then you may have noticed the tough
'tendons' that attach to it inside and which can be pulled like levers to operate the claw. The muscles attach
to these tendons which are internal extensions of the exoskeleton (and really constitute an internal
skeleton). The joints permit the rigid exoskeleton to move and consist of softer articulating membranes.
The arthropods were the first animals on Earth to truly colonise dry land. The jointed exoskeleton could
support their weight and the cuticle, with the addition of waterproofing waxy layers prevented dehydration.
The first land arthropods were myriapod-like animals: centipedes and millipedes, some as large as a
human, living in the forests of mosses. Like myriapods today they had many body segments, each bearing
a pair of legs, whilst the head developmentally was equivalent to several segments fused together, with the
limbs adapted as palps for feeling and tasting, antennae for smelling and mouthparts for eating. Over time,
some of their ancestors lost many of their legs as locomotion became refined, making do with just 8, as in
arachnids, or 6 as in insects (hexapods). Archaic insects are intermediate, Marchantia, for example, having
never evolved wings and still bearing rudimentary legs on the other segments and with palps that still look
like a 4th pair of legs.
Insect Legs
The arthropod leg can be divided into a number of segments, or podites, starting at the base and working
towards the foot these podites are: the cox, trochanter, femur, tibia, the tarsus composed of five segments
or tarsomeres, and finally the pretarsus, present as a pair of claws and sometimes also an adhesive pad.
These segments are variously modified in different insects. In the honeybee hindleg (metathoracic leg) for
example, the tibia contains the pollen basket for carrying pollen and the first basal-most tarsus segment,
the basitarsus, the pollen brush.
and is being used as a basis by robotocists to develop locomotion in their own machines!
The locomotive power for insects comes from the thorax and its appendage (legs and wings). The anatomy
of the thorax is illustrated above.
Insect locomotion is characterised by two main advancements: the jointed limbs characteristic of arthropods
that allowed the arthropods to colonise land and flight. The jointed limb of arthropods (arthropod meaning
'jointed leg') evolved in marine forms, such as crustaceans that are often amphibious, for example many
shore crabs. The tough exoskeleton supports the weight of the animal and also has internal projections to
which the muscles attach. If you have ever eaten a crab claw, then you may have noticed the tough
'tendons' that attach to it inside and which can be pulled like levers to operate the claw. The muscles attach
to these tendons which are internal extensions of the exoskeleton (and really constitute an internal
skeleton). The joints permit the rigid exoskeleton to move and consist of softer articulating membranes.
The arthropods were the first animals on Earth to truly colonise dry land. The jointed exoskeleton could
support their weight and the cuticle, with the addition of waterproofing waxy layers prevented dehydration.
The first land arthropods were myriapod-like animals: centipedes and millipedes, some as large as a
human, living in the forests of mosses. Like myriapods today they had many body segments, each bearing
a pair of legs, whilst the head developmentally was equivalent to several segments fused together, with the
limbs adapted as palps for feeling and tasting, antennae for smelling and mouthparts for eating. Over time,
some of their ancestors lost many of their legs as locomotion became refined, making do with just 8, as in
arachnids, or 6 as in insects (hexapods). Archaic insects are intermediate, Marchantia, for example, having
never evolved wings and still bearing rudimentary legs on the other segments and with palps that still look
like a 4th pair of legs.
Insect Legs
The arthropod leg can be divided into a number of segments, or podites, starting at the base and working
towards the foot these podites are: the cox, trochanter, femur, tibia, the tarsus composed of five segments
or tarsomeres, and finally the pretarsus, present as a pair of claws and sometimes also an adhesive pad.
These segments are variously modified in different insects. In the honeybee hindleg (metathoracic leg) for
example, the tibia contains the pollen basket for carrying pollen and the first basal-most tarsus segment,
the basitarsus, the pollen brush.
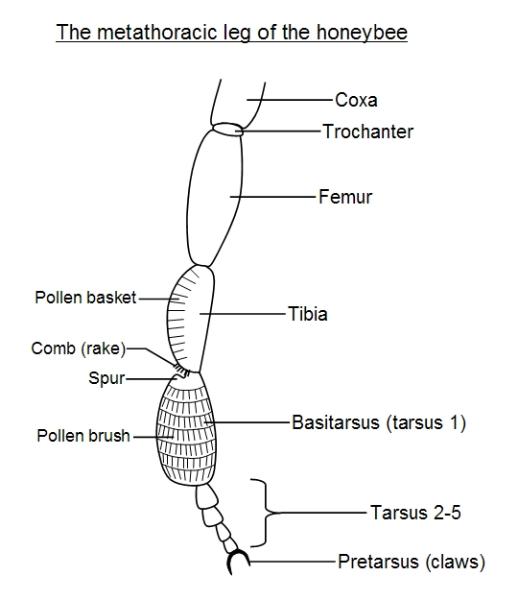
The honeybee uses its hindlegs to collect pollen. When it forages for nectar and pollen, pollen sticks to its
hairy body. In flight it grooms itself with its hindlegs, which have special rows of bristles forming the pollen
brush on the basal tarsal segment, which is enlarged. It then cleans the brush with a comb of bristles
situated at the bottom of the tibia, the left tibia combs the right leg pollen brush and vice versa. A
projection on the top of the basal tarsal segment, forming a spur, then pushes the pollen from the comb
into the pollen basket, which is also on the tibia.
In aquatic insects the legs may be modified into paddles or ores for swimming, and in the crustaceans we
see a bewildering array of modified limbs for performing different functions. The exoskeleton makes the
arthropod limb infinitely adaptable.
Typically the coxa and trochanter are short segments, and operated by muscles that insert inside the
thorax (extrinsic leg muscles). The tibia is operated by muscles in the femur above it (intrinsic leg
muscles). The femur itself generally moves little, being more-or-less fused to the trochanter. In a jumping
insect, such as the grasshopper, the femur is visibly greatly thickened and enlarged to accommodate the
powerful jumping muscles that operate the tibia.
Jumping Insects
A grasshopper can high-jump vertically upwards 45 cm (10 times its body length) and long-jump 90 cm
horizontally (20 times its body length). This is equivalent to a human high-jumping 60 feet (20 m) and
long-jumping 120 feet (40 m)! Insects are also strong for their size, being capable of carrying 2-3 times
their body weight, and up to 7-10 times their body weight in the case of ants. (Mind you human powerlifters
can comfortably lift 2-3 times their body weight or more, but they probably could not carry it far). This great
strength is actually due to their small size and it is unlikely that a grasshopper the size of a human could
really jump 60 feet! The strength of a muscle increases with the cross-sectional area or girth (thickness) of
the muscle, that is with the square of linear dimension, whilst weight increases with the cube of length.
Thus if we double the height of a human, keeping all proportions and other factors constant, then although
their strength would increase 4-fold, their weight would increase 8-fold and so their strength : weight ratio
would actually half! Thus, the great strength of insects is easily explained. In some cases an insect muscle
may consist of only one or a few muscle cells, as they don't need much motive force to effect great
strength for their size!
The thrust, or more specifically the force to weight ratio, of a grasshopper's jumping leg is about four times
that generated by a human leg. (In comparison, measurements indicate that the jumping power of a
chimpanzee leg is equal to that of a human leg, even though the muscle volume of the chimpanzee leg is
about half and chimpanzees typically lighter - chimpanzees are very good jumpers). This can be partly
explained by the greater relative strength of insects, due to their small size, but this results in an
acceleration rate that muscles cannot achieve unaided. It is the fact that the insect cuticle contains a very
elastic material called resilin, which is abundant in the cuticle of the legs of jumping insects, that enables
the insect leg to store energy. When preparing to jump the leg is locked in place as the powerful tibial
flexors (situated in the femur) contract, straining the cuticle of the locked leg which builds up elastic
energy. Suddenly, the cuticular lock is released and the leg springs straight, releasing the stored elastic
energy as the insect jumps. Resilin is almost perfectly elastic: 97% of the stored energy is released as
mechanical energy, only 3% is lost as unusable energy (heat and sound).
Insect Flight
The apterygotes are archaic insects that never evolved wings and are a kind of living fossil. A familiar
example is the domestic silverfish, which you may find scurrying around in kitchens and pantries, looking
for crumbs or bits of wood and paper to eat. Their fish-like serpentine motion and silvery bodies can make
them look like tiny fish swimming across the floor! This snakelike movement of the body increases their
stride-length and speed (since speed = stride length x stride rate). Amphibians deploy a similar tactic (see
locomotion).
The principles of flight have been discussed in our article on biorheology. The pterygotes are insects that
either have wings or evolved from winged ancestors. The wings are born, like the legs on the thorax.
Recall that the insect body can be divided into head, thorax and abdomen. The thorax consists of three
segments, the prothoracic segment nearest the head, the middle mesothoracic segment and the hindmost
metathoracic segment. The last two segments, the mesothoracic and metathoracic, each usually bear one
pair of wings. These wings are extensions of the cuticular plates of the thorax (the dorsal plate or tergite
and the side-plates (pleurites or pleura). [See the introductory page on insects for explanations of these
terms]. The anatomy of the flight muscles is illustrated below:
hairy body. In flight it grooms itself with its hindlegs, which have special rows of bristles forming the pollen
brush on the basal tarsal segment, which is enlarged. It then cleans the brush with a comb of bristles
situated at the bottom of the tibia, the left tibia combs the right leg pollen brush and vice versa. A
projection on the top of the basal tarsal segment, forming a spur, then pushes the pollen from the comb
into the pollen basket, which is also on the tibia.
In aquatic insects the legs may be modified into paddles or ores for swimming, and in the crustaceans we
see a bewildering array of modified limbs for performing different functions. The exoskeleton makes the
arthropod limb infinitely adaptable.
Typically the coxa and trochanter are short segments, and operated by muscles that insert inside the
thorax (extrinsic leg muscles). The tibia is operated by muscles in the femur above it (intrinsic leg
muscles). The femur itself generally moves little, being more-or-less fused to the trochanter. In a jumping
insect, such as the grasshopper, the femur is visibly greatly thickened and enlarged to accommodate the
powerful jumping muscles that operate the tibia.
Jumping Insects
A grasshopper can high-jump vertically upwards 45 cm (10 times its body length) and long-jump 90 cm
horizontally (20 times its body length). This is equivalent to a human high-jumping 60 feet (20 m) and
long-jumping 120 feet (40 m)! Insects are also strong for their size, being capable of carrying 2-3 times
their body weight, and up to 7-10 times their body weight in the case of ants. (Mind you human powerlifters
can comfortably lift 2-3 times their body weight or more, but they probably could not carry it far). This great
strength is actually due to their small size and it is unlikely that a grasshopper the size of a human could
really jump 60 feet! The strength of a muscle increases with the cross-sectional area or girth (thickness) of
the muscle, that is with the square of linear dimension, whilst weight increases with the cube of length.
Thus if we double the height of a human, keeping all proportions and other factors constant, then although
their strength would increase 4-fold, their weight would increase 8-fold and so their strength : weight ratio
would actually half! Thus, the great strength of insects is easily explained. In some cases an insect muscle
may consist of only one or a few muscle cells, as they don't need much motive force to effect great
strength for their size!
The thrust, or more specifically the force to weight ratio, of a grasshopper's jumping leg is about four times
that generated by a human leg. (In comparison, measurements indicate that the jumping power of a
chimpanzee leg is equal to that of a human leg, even though the muscle volume of the chimpanzee leg is
about half and chimpanzees typically lighter - chimpanzees are very good jumpers). This can be partly
explained by the greater relative strength of insects, due to their small size, but this results in an
acceleration rate that muscles cannot achieve unaided. It is the fact that the insect cuticle contains a very
elastic material called resilin, which is abundant in the cuticle of the legs of jumping insects, that enables
the insect leg to store energy. When preparing to jump the leg is locked in place as the powerful tibial
flexors (situated in the femur) contract, straining the cuticle of the locked leg which builds up elastic
energy. Suddenly, the cuticular lock is released and the leg springs straight, releasing the stored elastic
energy as the insect jumps. Resilin is almost perfectly elastic: 97% of the stored energy is released as
mechanical energy, only 3% is lost as unusable energy (heat and sound).
Insect Flight
The apterygotes are archaic insects that never evolved wings and are a kind of living fossil. A familiar
example is the domestic silverfish, which you may find scurrying around in kitchens and pantries, looking
for crumbs or bits of wood and paper to eat. Their fish-like serpentine motion and silvery bodies can make
them look like tiny fish swimming across the floor! This snakelike movement of the body increases their
stride-length and speed (since speed = stride length x stride rate). Amphibians deploy a similar tactic (see
locomotion).
The principles of flight have been discussed in our article on biorheology. The pterygotes are insects that
either have wings or evolved from winged ancestors. The wings are born, like the legs on the thorax.
Recall that the insect body can be divided into head, thorax and abdomen. The thorax consists of three
segments, the prothoracic segment nearest the head, the middle mesothoracic segment and the hindmost
metathoracic segment. The last two segments, the mesothoracic and metathoracic, each usually bear one
pair of wings. These wings are extensions of the cuticular plates of the thorax (the dorsal plate or tergite
and the side-plates (pleurites or pleura). [See the introductory page on insects for explanations of these
terms]. The anatomy of the flight muscles is illustrated below:
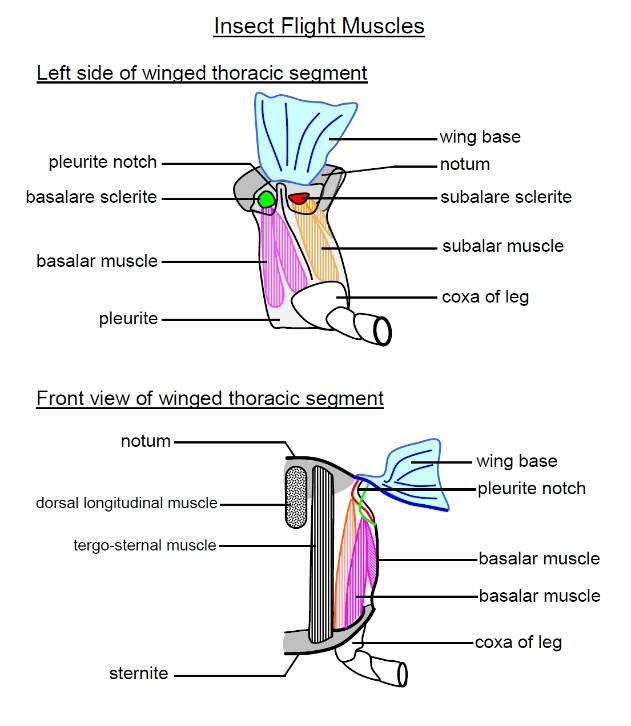
Each thoracic segment is encased in four groups of cuticular plates: the notum (tergum) on the back, the
sternum on the front and the pleura (singular pleuron) on the sides. These four regions of the cutcicle are
made up of cuticular plates, called respectably the tergites, sternites and pleurites, and softer artioculating
membranes that connect the regions together. The flight muscles attach to certain of these plates or
sclerites.
There are two sets of flight muscles - direct and indirect muscles. The direct muscles attach to the wing
base, or more specifically to sclerites (cuticular plates of the exoskeleton) in the pleura (the groups of
cuticular plates making up the sides of the insect) that then attach to the wing base. The base of the wing
attaches to the notum and pleuron. The notum (or tergum) is the plate that forms the insect's back and the
pleuron its side.
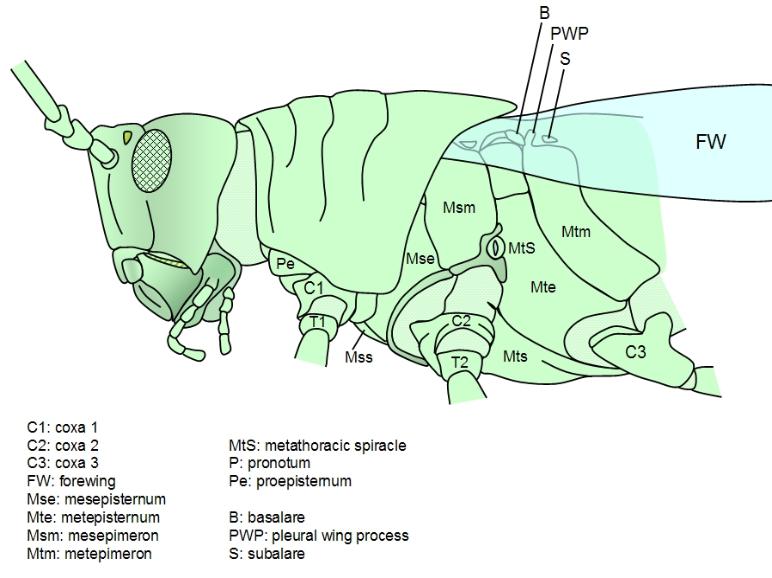
Direct muscles. The direct muscles attach to sclerites in the pleura at both ends. There are two paired sets
of these muscles: the basalar muscles and the subalar muscles. The wing hinge pivots on a projection of
the main pleurite, called the pleurite notch or pleural wing process (PWP). The basalar muscles attach
just anterior of this pivot point, to a small pleurite called the basalare sclerite. The subalare muscles attach
just posterior to this pivot, to a small pleural sclerite called the subalare sclerite. In dragonflies, the wings
do not need to move very fast, as these insects are large, and these direct flight muscles provide the motive
power, lowering the wing by contracting. However, in most insects the direct flight muscles are only used to
tilt the wing. The wing root contains a number of small cuticular plates, called axillary sclerites, the second
axillary sclerite articulates with the PWP and connects to the subalare. the subalare is hinged to the main
pleurite just behind the PWP, the basalare in front.
When most insects fly, the wing tips trace a figure-of-eight - tipping the leading edge downwards as the
wing moves forwards and downwards. This is achieved by contracting the basalar muscles, pulling on the
basalare sclerite, depressing it and transferring the strain to the wing base, due to the elasticity of this
region of the cuticle (due to resilin again). This forward-downstroke is the main power stroke. The wing
begins the downward loop with a high angle of attack, but as the leading edge tilts downwards, the wing
momentarily becomes horizontal, in the middle of the stroke, minimising the angle of attack. At this point the
wing is moving fastest, and reducing the angle of attack prevents stalling. When the wings move upwards
and backwards, during the recovery stroke, the leading edge tips backwards; this is achieved by contracting
the subalare muscles, lifting the leading edge of the wing about its pivot. The wing is rotated again at the top
of the recovery stroke, restoring the maximum angle of attack just before the downstroke begins.
Indirect muscles. These provide most of the motive power in most insects, whilst the direct flight muscles
rotate the wings, controlling the angle-of-attack. They consist of two antagonistic pairs of muscles: the dorsal
longitudinal muscles and the sterno-tergal (or dorso-ventral) muscles. [These muscles occur in most
segments of the insect body, thoracic and abdominal, where they allow the segments to change shape and
telescope in and out of one-anotehr to some degree, but are especially well-developed for flight in the
meso- and metathoracic segments, that is the second and third segments which bear the wings).
The dorsal longitudinal muscle pair run lengthwise along the top (dorsal side) of each (winged) thoracic
segment, attaching to the phragmata, which lie just beneath the tergal antecostal sutures, at both ends. The
antecostal suture is an intersegmental ring (visible from the outside as the junction between segments),
where the tergite (and sternite) curve inwards at the ends of each segment. The tergite of each segment
curves down, underneath the back of the tergite in-front of it (rather like subducting tectonic plates) at the
front. This forms an internal cuticular ridge, running transverse (across the thorax) at the front of each
segment, called a phragma (plural phragmata). In each segment the dorsal longitudinals attach to the
phragma of its own segment at the front, and to the phragma of the segment behind it at the back.
Contraction of these muscles will shorten (and deepen) the thoracic segment slightly. This raises the notum,
causing the wings to pivot on elastic hinges and move downwards (wing depression).
The tergo-sternal muscle pair (or sterno-tergal or dorso-ventral muscles, etc.) run vertically, joining to the
sternite at the bottom and to the tergite at the top. Contraction of these muscles flattens the thoracic
segment (and lengthens it) slightly. This pulls the notum down slightly, causing the wings to pivot on elastic
hinges and lift upwards (wing elevation).
In many insects, the notum actually switches between a depressed state and a raised state quite suddenly,
as the cuticle clicks in place - the so-called click mechanism. This means that as the indirect flight muscles
contract, strain builds up in the elastic cuticle, storing energy that is released when the notum suddenly click
into a new position, releasing the built-up elastic energy which helps power the wings. However, later authors
queried the validity of the click mechanism and several alternative, but similar, mechanisms have been
proposed, one of the most promising for flies is described in more detail here: indirect flight muscle
mechanism.
Summary of muscle action:
Insects can achieve flight speeds of up to 25 mph (40 kph). A dragonfly, for example, can cruise at 18 mph,
a honeybee at 14 mph. A dragonfly will beat its wings at 20-30 beats per second (bps or Hertz, Hz), a
housefly (Musca) at 100 bps, a mosquito at 300-600 bps and Forcipomyia at 1000 bps, which is extremely
fast! The faster of these exceed the flicker fusion frequency of the human eye (50-60 Hz) at which motions
blurs, and so the individual wing beats become impossible to see without playing slowed down recordings.
Control of the flight muscles
Nerves can conduct impulses no faster than about 30 pulses per second. How then can these slow nerves
control such fast muscles? In the dragonfly, there is no problem, one nerve impusle can bring about one
wing beat - the flight muscles are controlled neurogenically. The muscle contractions and nerve pulses are
synchronous. In insects that beat their wings much faster, however, nerve impulses simply start and stop
the machine, once the wings get going the indirect flight muscles are alternately stretched as they relax - the
dorsal longitudinals stretch when the tergo-sternal contract, and vice versa. When these muscles are
stretched, this stretching triggers their subsequent contraction, so cycles of relaxing-stretching-contracting
can occur rapidly, without having to wait for the nervous system to keep up! The nerve impulses and muscle
contractions are then asynchronous.
Flight Muscle Physiology
The direct flight muscles are very well-developed and resemble vertebrate muscle in many ways. The
indirect flight muscles, however, are exceptional! They are extremely powerful. Vertebrate biologists may
have heard that hummingbird flight muscles are the most powerful in the animal kingdom, power referring to
work rate, this is partially correct. In fact they tie for the title of most powerful muscles with insect indirect
flight muscles! Both muscle types have very similar power outputs. [Old reports that insect flight muscles
could generate the power output equivalent of a piston-driven combustion-engine were erroneous,
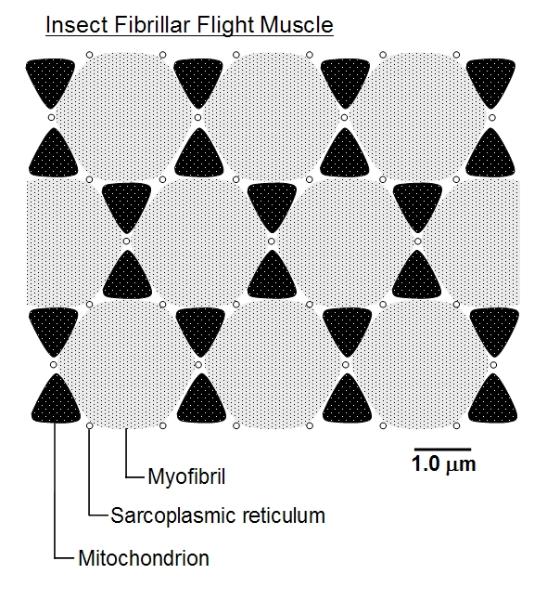
however.] These indirect flight muscles have very characteristic structure under the electron microscope.
Not only are they striated, like vertebrate muscles, but each cell or muscle fibre is packed with contractile
motor proteins and has a well-developed sarcoplasmic reticulum (membranous sacs that store calcium
ions which signal and initiate contraction) and a regular array of well-developed mitochondria. The whole
looks remarkably regular and semi-crystalline, as illustrated below. They are thus called fibrillar muscles.
sternum on the front and the pleura (singular pleuron) on the sides. These four regions of the cutcicle are
made up of cuticular plates, called respectably the tergites, sternites and pleurites, and softer artioculating
membranes that connect the regions together. The flight muscles attach to certain of these plates or
sclerites.
There are two sets of flight muscles - direct and indirect muscles. The direct muscles attach to the wing
base, or more specifically to sclerites (cuticular plates of the exoskeleton) in the pleura (the groups of
cuticular plates making up the sides of the insect) that then attach to the wing base. The base of the wing
attaches to the notum and pleuron. The notum (or tergum) is the plate that forms the insect's back and the
pleuron its side.
Direct muscles. The direct muscles attach to sclerites in the pleura at both ends. There are two paired sets
of these muscles: the basalar muscles and the subalar muscles. The wing hinge pivots on a projection of
the main pleurite, called the pleurite notch or pleural wing process (PWP). The basalar muscles attach
just anterior of this pivot point, to a small pleurite called the basalare sclerite. The subalare muscles attach
just posterior to this pivot, to a small pleural sclerite called the subalare sclerite. In dragonflies, the wings
do not need to move very fast, as these insects are large, and these direct flight muscles provide the motive
power, lowering the wing by contracting. However, in most insects the direct flight muscles are only used to
tilt the wing. The wing root contains a number of small cuticular plates, called axillary sclerites, the second
axillary sclerite articulates with the PWP and connects to the subalare. the subalare is hinged to the main
pleurite just behind the PWP, the basalare in front.
When most insects fly, the wing tips trace a figure-of-eight - tipping the leading edge downwards as the
wing moves forwards and downwards. This is achieved by contracting the basalar muscles, pulling on the
basalare sclerite, depressing it and transferring the strain to the wing base, due to the elasticity of this
region of the cuticle (due to resilin again). This forward-downstroke is the main power stroke. The wing
begins the downward loop with a high angle of attack, but as the leading edge tilts downwards, the wing
momentarily becomes horizontal, in the middle of the stroke, minimising the angle of attack. At this point the
wing is moving fastest, and reducing the angle of attack prevents stalling. When the wings move upwards
and backwards, during the recovery stroke, the leading edge tips backwards; this is achieved by contracting
the subalare muscles, lifting the leading edge of the wing about its pivot. The wing is rotated again at the top
of the recovery stroke, restoring the maximum angle of attack just before the downstroke begins.
Indirect muscles. These provide most of the motive power in most insects, whilst the direct flight muscles
rotate the wings, controlling the angle-of-attack. They consist of two antagonistic pairs of muscles: the dorsal
longitudinal muscles and the sterno-tergal (or dorso-ventral) muscles. [These muscles occur in most
segments of the insect body, thoracic and abdominal, where they allow the segments to change shape and
telescope in and out of one-anotehr to some degree, but are especially well-developed for flight in the
meso- and metathoracic segments, that is the second and third segments which bear the wings).
The dorsal longitudinal muscle pair run lengthwise along the top (dorsal side) of each (winged) thoracic
segment, attaching to the phragmata, which lie just beneath the tergal antecostal sutures, at both ends. The
antecostal suture is an intersegmental ring (visible from the outside as the junction between segments),
where the tergite (and sternite) curve inwards at the ends of each segment. The tergite of each segment
curves down, underneath the back of the tergite in-front of it (rather like subducting tectonic plates) at the
front. This forms an internal cuticular ridge, running transverse (across the thorax) at the front of each
segment, called a phragma (plural phragmata). In each segment the dorsal longitudinals attach to the
phragma of its own segment at the front, and to the phragma of the segment behind it at the back.
Contraction of these muscles will shorten (and deepen) the thoracic segment slightly. This raises the notum,
causing the wings to pivot on elastic hinges and move downwards (wing depression).
The tergo-sternal muscle pair (or sterno-tergal or dorso-ventral muscles, etc.) run vertically, joining to the
sternite at the bottom and to the tergite at the top. Contraction of these muscles flattens the thoracic
segment (and lengthens it) slightly. This pulls the notum down slightly, causing the wings to pivot on elastic
hinges and lift upwards (wing elevation).
In many insects, the notum actually switches between a depressed state and a raised state quite suddenly,
as the cuticle clicks in place - the so-called click mechanism. This means that as the indirect flight muscles
contract, strain builds up in the elastic cuticle, storing energy that is released when the notum suddenly click
into a new position, releasing the built-up elastic energy which helps power the wings. However, later authors
queried the validity of the click mechanism and several alternative, but similar, mechanisms have been
proposed, one of the most promising for flies is described in more detail here: indirect flight muscle
mechanism.
Summary of muscle action:
- During the downstroke: the tergo-sternal muscles relax as the dorsal longitudinals contract,
depressing the wing and generating lift as the wings move forwards and downwards. Basalar muscles
contract, as the subalar relax, tilting the leading edge of the wing downwards (to prevent stalling).
- During the upstroke: the dorsal longitudinal muscles relax as the tergo-sternals contract, lowering the
notum and raising the wings during the recovering stroke as the wings move backwards and upwards.
subalar muscle contract as basalar muscles relax, raising the leading edge of the wing.
Insects can achieve flight speeds of up to 25 mph (40 kph). A dragonfly, for example, can cruise at 18 mph,
a honeybee at 14 mph. A dragonfly will beat its wings at 20-30 beats per second (bps or Hertz, Hz), a
housefly (Musca) at 100 bps, a mosquito at 300-600 bps and Forcipomyia at 1000 bps, which is extremely
fast! The faster of these exceed the flicker fusion frequency of the human eye (50-60 Hz) at which motions
blurs, and so the individual wing beats become impossible to see without playing slowed down recordings.
Control of the flight muscles
Nerves can conduct impulses no faster than about 30 pulses per second. How then can these slow nerves
control such fast muscles? In the dragonfly, there is no problem, one nerve impusle can bring about one
wing beat - the flight muscles are controlled neurogenically. The muscle contractions and nerve pulses are
synchronous. In insects that beat their wings much faster, however, nerve impulses simply start and stop
the machine, once the wings get going the indirect flight muscles are alternately stretched as they relax - the
dorsal longitudinals stretch when the tergo-sternal contract, and vice versa. When these muscles are
stretched, this stretching triggers their subsequent contraction, so cycles of relaxing-stretching-contracting
can occur rapidly, without having to wait for the nervous system to keep up! The nerve impulses and muscle
contractions are then asynchronous.
Flight Muscle Physiology
The direct flight muscles are very well-developed and resemble vertebrate muscle in many ways. The
indirect flight muscles, however, are exceptional! They are extremely powerful. Vertebrate biologists may
have heard that hummingbird flight muscles are the most powerful in the animal kingdom, power referring to
work rate, this is partially correct. In fact they tie for the title of most powerful muscles with insect indirect
flight muscles! Both muscle types have very similar power outputs. [Old reports that insect flight muscles
could generate the power output equivalent of a piston-driven combustion-engine were erroneous,
however.] These indirect flight muscles have very characteristic structure under the electron microscope.
Not only are they striated, like vertebrate muscles, but each cell or muscle fibre is packed with contractile
motor proteins and has a well-developed sarcoplasmic reticulum (membranous sacs that store calcium
ions which signal and initiate contraction) and a regular array of well-developed mitochondria. The whole
looks remarkably regular and semi-crystalline, as illustrated below. They are thus called fibrillar muscles.
Suggested Reading
http://www.zoo.cam.ac.uk/zoostaff/ellington/aerodynamics.html
Anderson, M. and Finlayson, L.H. 1976. The effect of exercise on the growth of mitochondria and myofibrils in the
flight muscles of the tsetse fly, Glossina morsitans. J. Morph. 150: 321–326.
Anderson, M. and Finlayson, L.H. Ultrastructural changes during growth of the flight muscles in the adult tsetse fly,
Glossina austeni.
Brackenbury, J. 1996. Novel locomotory mechanisms in caterpillars: life-line climbing in Epinotia abbreviana
(Torticidae) and Yponomeuta padella (Yponomeutidae). Phys. Ent. 21: 7-14.
Evans, H.E. 1984. Insect Biology: a textbook of entomology. Addison wesley (pub.).
Federle, W., Brainerd, E. L., McMahon, T. A. and Holldobler, B. 2001. Biomechanics of the movable pretarsal
adhesive organ in ants and bees. PNAS 98: 6215–6220.
Gettrup. E. 1966. Sensory regulation of wing twisting in locusts. J. Exp. Biol. 44: 1-16.
Wigglesworth, 1972. The principles of insect physiology, 7th ed. Cambridge University Press.
Wilson, D. M. 1961. The central nervous control of light in a locust. J. Exp. Biol. 38: 47I - 490.
http://www.zoo.cam.ac.uk/zoostaff/ellington/aerodynamics.html
Anderson, M. and Finlayson, L.H. 1976. The effect of exercise on the growth of mitochondria and myofibrils in the
flight muscles of the tsetse fly, Glossina morsitans. J. Morph. 150: 321–326.
Anderson, M. and Finlayson, L.H. Ultrastructural changes during growth of the flight muscles in the adult tsetse fly,
Glossina austeni.
Brackenbury, J. 1996. Novel locomotory mechanisms in caterpillars: life-line climbing in Epinotia abbreviana
(Torticidae) and Yponomeuta padella (Yponomeutidae). Phys. Ent. 21: 7-14.
Evans, H.E. 1984. Insect Biology: a textbook of entomology. Addison wesley (pub.).
Federle, W., Brainerd, E. L., McMahon, T. A. and Holldobler, B. 2001. Biomechanics of the movable pretarsal
adhesive organ in ants and bees. PNAS 98: 6215–6220.
Gettrup. E. 1966. Sensory regulation of wing twisting in locusts. J. Exp. Biol. 44: 1-16.
Wigglesworth, 1972. The principles of insect physiology, 7th ed. Cambridge University Press.
Wilson, D. M. 1961. The central nervous control of light in a locust. J. Exp. Biol. 38: 47I - 490.
| Did you know? Just as humans can build up their muscles in the gym, so insect muscles develop with increased exercise. experiments have shown that if flies are reared in captivity, then periodically banging the cage to make them fly dramatically increases their muscle development! Indeed, regular exercise is essential for them to develop proper fibrillar flight muscles. |
This page is under construction...
Please report any errors to botrejectsinc@cronodon.com
The role of vortices in insect flight
Insects do not generate all their lift and thrust simply by pushing air downwards and backwards as they
beat their wings. The insect wing also acts as an aerofoil as its lices through the air (which is why angle of
attack is so important). This means that as air flows over its surface it is set into circulation around the
wing. The inevitable result is that vortices are shed from the tips of insect wings and these vortices (or
rather the circulation that causes them) are essential in lift generation see the page on biorheology for
details of flight mechanics.
Other flight mechanisms
Some of the smallest insects have wings that are not aerofoils, but simple groups of bristles! These seem
to work more like oars, rowing the insect through the air. This happens because for a very small insect the
Reynold's number is very low. Reynold's number is the ratio of inertial forces (due to bulk fluid flow) to
viscous forces (due to the 'stickiness' of a fluid). At very small scales viscous forces dominate and air
becomes more like water (and water like treacle)!
The tiny wasp Encarsia formosa generates lift by a clap-and-fling mechanism. The insect alternately
claps its wings together behind its back, and then flings them apart. When they part, they separate first
along the leading edge, causing air to rush into the space between them. This causes air to circulate
around the wings, again generating vortices and lift, but without the normal aerofoil mechanism.
The Insect Wing
Evidence from the fossil record indicates that wings evolved from lateral outgrowths of the nota (tergites) of
the thoracic segments. It is thought that these were used to absorb heat, perhaps when the insect as
basking in the sun at dawn. Insects are largely poikilothermic ectotherms, meaning that they do not
regulate their body temperature by generating internal heat (though this is a generalisation and some
insects vibrate their wing muscles prior to flight to generate the necessary heat), in other words they are
'cold blooded'. Absorbing heat through these plates which may have been well-supplied with blood and
have a large surface area would warm the leg muscles of the thorax, ready for action. They may also have
been used in respiration, absorbing oxygen for the leg muscles. They could also have been used in visual
displays, perhaps in courtship and may have been brightly coloured.
It would appear that the wings of modern insects can still perform these functions. The wings are thin
membranous 'gossamer' sheets of little more than a double layer of epithelial cells covered by chitinous
secretion. This structure is supported by a series of veins, each containing a trachea and perhaps a nerve
bathed in haemolymph (insect 'blood') which runs in the channel between the trachea and the epithelium
wall. There is evidence that insect blood facilitates the transport of oxygen, though this is mainly carried out
by the tracheal system and the haemolymph often runs in channels close to the tracheae. The large wings
of lepidopterans are thought to have a significant role in absorbing oxygen from the air. Beating the wings
must assist this by circulating the air around the wings.
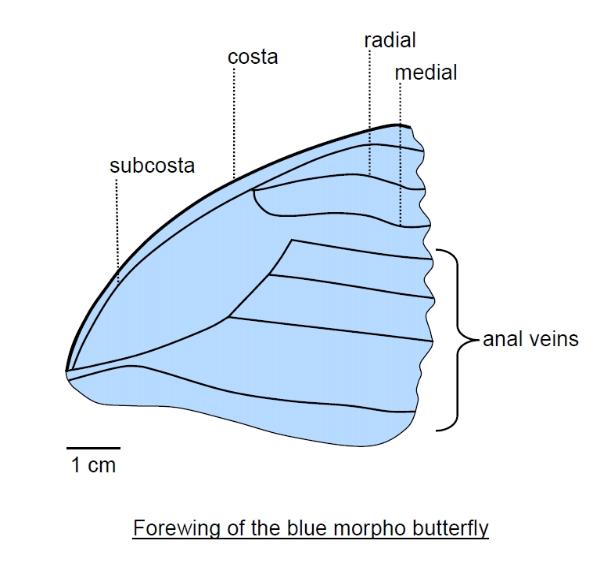
The pattern of veins (venation) of the insect wing varies considerably among species, but there is a
general pattern. Along the leading margin (costal margin) of a wing runs a prominent vein called the costa.
Behind this is the subcosta and then a pair of veins that branch from a common source, called the radial
and medial veins. Behind this is a separate group of veins called the anal veins (as they span the rear
portion of the wing).
Please report any errors to botrejectsinc@cronodon.com
The role of vortices in insect flight
Insects do not generate all their lift and thrust simply by pushing air downwards and backwards as they
beat their wings. The insect wing also acts as an aerofoil as its lices through the air (which is why angle of
attack is so important). This means that as air flows over its surface it is set into circulation around the
wing. The inevitable result is that vortices are shed from the tips of insect wings and these vortices (or
rather the circulation that causes them) are essential in lift generation see the page on biorheology for
details of flight mechanics.
Other flight mechanisms
Some of the smallest insects have wings that are not aerofoils, but simple groups of bristles! These seem
to work more like oars, rowing the insect through the air. This happens because for a very small insect the
Reynold's number is very low. Reynold's number is the ratio of inertial forces (due to bulk fluid flow) to
viscous forces (due to the 'stickiness' of a fluid). At very small scales viscous forces dominate and air
becomes more like water (and water like treacle)!
The tiny wasp Encarsia formosa generates lift by a clap-and-fling mechanism. The insect alternately
claps its wings together behind its back, and then flings them apart. When they part, they separate first
along the leading edge, causing air to rush into the space between them. This causes air to circulate
around the wings, again generating vortices and lift, but without the normal aerofoil mechanism.
The Insect Wing
Evidence from the fossil record indicates that wings evolved from lateral outgrowths of the nota (tergites) of
the thoracic segments. It is thought that these were used to absorb heat, perhaps when the insect as
basking in the sun at dawn. Insects are largely poikilothermic ectotherms, meaning that they do not
regulate their body temperature by generating internal heat (though this is a generalisation and some
insects vibrate their wing muscles prior to flight to generate the necessary heat), in other words they are
'cold blooded'. Absorbing heat through these plates which may have been well-supplied with blood and
have a large surface area would warm the leg muscles of the thorax, ready for action. They may also have
been used in respiration, absorbing oxygen for the leg muscles. They could also have been used in visual
displays, perhaps in courtship and may have been brightly coloured.
It would appear that the wings of modern insects can still perform these functions. The wings are thin
membranous 'gossamer' sheets of little more than a double layer of epithelial cells covered by chitinous
secretion. This structure is supported by a series of veins, each containing a trachea and perhaps a nerve
bathed in haemolymph (insect 'blood') which runs in the channel between the trachea and the epithelium
wall. There is evidence that insect blood facilitates the transport of oxygen, though this is mainly carried out
by the tracheal system and the haemolymph often runs in channels close to the tracheae. The large wings
of lepidopterans are thought to have a significant role in absorbing oxygen from the air. Beating the wings
must assist this by circulating the air around the wings.
The pattern of veins (venation) of the insect wing varies considerably among species, but there is a
general pattern. Along the leading margin (costal margin) of a wing runs a prominent vein called the costa.
Behind this is the subcosta and then a pair of veins that branch from a common source, called the radial
and medial veins. Behind this is a separate group of veins called the anal veins (as they span the rear
portion of the wing).
Walking and Running in Insects
The standard explanation of insect walking is as follows. The insect maximises stability by keeping a
supporting tripod of three feet on the ground at any one time, the front and hind-leg of one side and the
middle leg on the other, whilst the other three are lifted in the air stepping forward to form the next tripod.
With each step the insect falls from one tripod to the next, such that there is an alternation from a tripod
formed by the fore and hind-legs of the right side with the middle leg of the left side and a tripod formed by
the middle leg of the right-side and the fore and hind-legs of the left side and so on. In each step the
hindleg on the ground provides most of the thrust, causing the insect to pivot to the side. (When the right
hindleg is pushing on the ground it pivots to the right, and when the left hindleg is pushing it pivots to the
left). Thus, the insect moves in a zig-zag path as it advances. Keeping at least three feet on the ground at
any one time gives optimum stability, as a tripod is a very stable structure, preventing the insect from
falling over. This stability is important as insects, despite offering less surface to the wind, can be blown a
few millimetres off course by strong winds and so they have to make sure they are do not fall. This stability
minimises on central nervous system processing - in bipeds, for example, the brain has to do much more
processing to ensure that balance is maintained.
The standard explanation of insect walking is as follows. The insect maximises stability by keeping a
supporting tripod of three feet on the ground at any one time, the front and hind-leg of one side and the
middle leg on the other, whilst the other three are lifted in the air stepping forward to form the next tripod.
With each step the insect falls from one tripod to the next, such that there is an alternation from a tripod
formed by the fore and hind-legs of the right side with the middle leg of the left side and a tripod formed by
the middle leg of the right-side and the fore and hind-legs of the left side and so on. In each step the
hindleg on the ground provides most of the thrust, causing the insect to pivot to the side. (When the right
hindleg is pushing on the ground it pivots to the right, and when the left hindleg is pushing it pivots to the
left). Thus, the insect moves in a zig-zag path as it advances. Keeping at least three feet on the ground at
any one time gives optimum stability, as a tripod is a very stable structure, preventing the insect from
falling over. This stability is important as insects, despite offering less surface to the wind, can be blown a
few millimetres off course by strong winds and so they have to make sure they are do not fall. This stability
minimises on central nervous system processing - in bipeds, for example, the brain has to do much more
processing to ensure that balance is maintained.
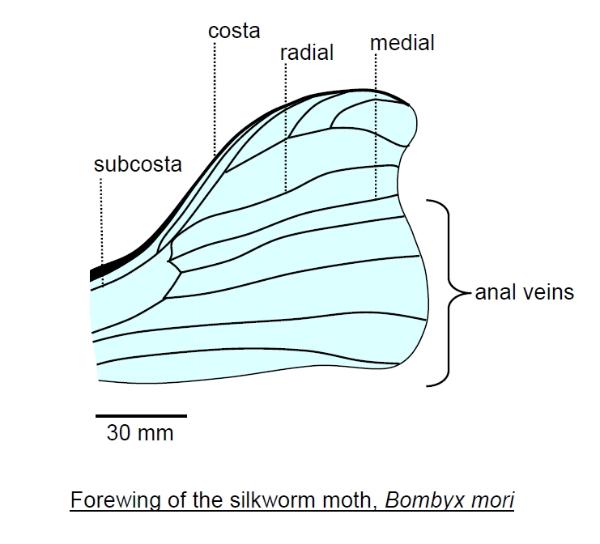
Insect 'blood', properly called haemolymph, circulates around the wing veins, maintaining the wing which
would otherwise become dry and brittle. Accessory hearts or muscular pumps are often present either at the
root of the veins at the wing base or along the course of the veins, helping to pump the haemolymph around
the wing. The main pumps, however, are located in the thorax. The haemolymph typically enters the wing at
the costa, travels around the margin of the wing and then returns along the more posterior veins, such as
the anal veins. Remember that insects have an open circulatory system, so there are regions in which
haemolymph flows rather loosely in haemolymph spaces rather than vessels, as between the costa and anal
veins.
would otherwise become dry and brittle. Accessory hearts or muscular pumps are often present either at the
root of the veins at the wing base or along the course of the veins, helping to pump the haemolymph around
the wing. The main pumps, however, are located in the thorax. The haemolymph typically enters the wing at
the costa, travels around the margin of the wing and then returns along the more posterior veins, such as
the anal veins. Remember that insects have an open circulatory system, so there are regions in which
haemolymph flows rather loosely in haemolymph spaces rather than vessels, as between the costa and anal
veins.
The wing is equipped with sensors. Bristles, presumably tactile, typically line the margin, and clusters of tiny
dome-shaped campaniform sensilla. A campaniform sensillum consists of a small cuticular dome,
sometimes at the bottom of a shallow pit, with a mechanosensitive dendrite underneath. The dendrite is a
process from a modified epithelial cell. The number and arrangement of these sensilla depends on species.
They are usually found on the undersurface of the wing. In the locust, Schistocerca, there are about 95 on
on the undersurface of the costa, one group of about 20 near the wing base (arranged in a fan-shaped
array) and the second group of about 60 towards the wing tip. The cockroach has only 20-40 campaniform
sensilla, mostly on the forewings, whilst good fliers, like true flies, bees and wasps, may have 6-7 times as
many as the locust, arranged in 6 groups or so. The campaniform sensilla are proprioceptors, detecting
flexing and torque in the wing cuticle. Strains in the wing cause the domes to reversibly buckle, activating
the sensory dendrite underneath. They can thus detect changes in position of the wings. Their domes are
elliptical, rather than round, and they are aligned in such a way that they are most sensitive to wing twisting
(pronation and supination) such as when the wing changes its angle of attack through the wing stroke-cycle.
The main pattern of wing movement is generated by the central nervous system. Each segment of the
insect body has a pair of ganglia that are more-or-less fused into a single ganglion, situated ventrally and
connected to the ganglia in other segments by the double ventral nerve cord. The ganglia of some adjacent
segments may also be fused together, especially in the head and abdomen. These act as local processors
or 'brains'. The ganglia of the mesothoracic and metathoracic segments, the wing-bearing segments, are
chiefly responsible for controlling the wings. The brain is not directly required. Some of the anterior
abdominal ganglia may also contribute. These form a central-pattern generator that generates the
correct pattern of firing to move the wings even in the absence of sensory input. However, sensory input,
chiefly from the campaniform sensilla of the wings is essential to maintain the correct frequency of wingbeat
and also in the control fine movements of the wing, such as wing twisting.
The antennae are also involved in regulating flight speed. Both the strain acting on them by the moving air,
and the vibrations this causes, is sensed by Johnston's organ in the base of each antenna, giving a
measure of air speed. Initiation of flight can be brought about by loss of contact between the feet (tarsi) and
the ground, by pinching the abdomen, but this soon stops unless air is kept blowing on sensory bristles on
the insect's face. Thus, the head may not be needed to initiate flight, but it has a role in maintaining it and
in regulating speed.
The importamce of feedback from the sensory organs is illustrated by the observation that many insects
can continue to fly with one of their four wings missing. Dragonflies are especially noted for this, being able
to compensate and maintain stability in the air despite losing one of their hindwings, though their speed will
be reduced due to a loss of thrust.
The hindwings typically provide most of the thrust, each hindwing providing about 35% of the thrust in a
flying locust. The forewings may be toughened top provide protection for the hindwings, forming
parchment-like tegmina (singular tegmen), or in the case of beetle the very hard elytra (singular elytron)
or wing-covers. In many true bugs, Hemiptera, only the basal part of the forewing is thickened and these
are called hemelytra. In true flies, Diptera, the hindwings are reduced to the small club-like halteres,
which beat out of phase with the forewings and act as sensory gyroscopes to maintain flight-stability (they
are loaded with campaniform sensilla).
It is important that the forewings do not interfere with the hindwings during flight. In locusts, cocokroaches
and dragonflies the four wings are kept separate but each pair beats out of phase with the other. For
example in the locust the wings beat about 17 times per second and the forewings lead the hindwings by
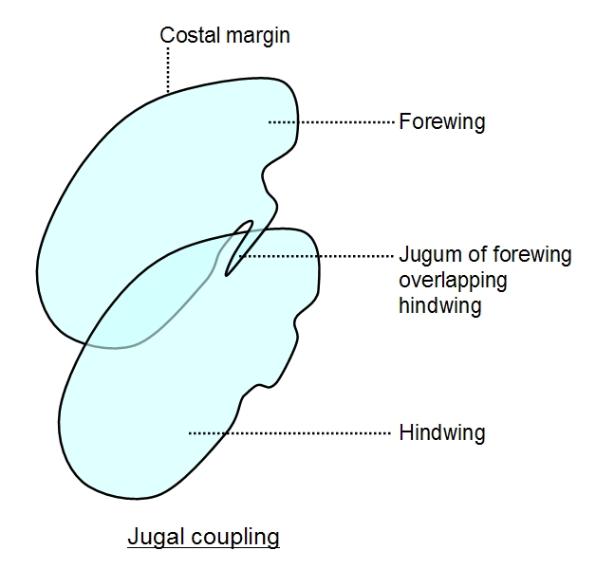
about 6-8 millseconds. Some insects solve the problem by coupling the wings together. For example some
moths and butterflies have jugal coupling, in which a toothlike process extending from the hind-margin
(anal margin) of the forewing, called the jugum, fold under the hindwing in flight, so that forewings and
hindwings are locked together and function as a single pair of wings. This is much like cutting a slit in the
margin of a sheet of paper and slotting another sheet into it. In some moths, the mechanism used is
frenulo-retinacular coupling, in which a spinelike frenulum on the fore-margin (costal margin) of the
hindwing hooks under the loop-like retinaculum on the underside of the forewing. Amplexiform
coupling, seen in some butterflies, involves no special hooks, but the wings overlap significantly.
dome-shaped campaniform sensilla. A campaniform sensillum consists of a small cuticular dome,
sometimes at the bottom of a shallow pit, with a mechanosensitive dendrite underneath. The dendrite is a
process from a modified epithelial cell. The number and arrangement of these sensilla depends on species.
They are usually found on the undersurface of the wing. In the locust, Schistocerca, there are about 95 on
on the undersurface of the costa, one group of about 20 near the wing base (arranged in a fan-shaped
array) and the second group of about 60 towards the wing tip. The cockroach has only 20-40 campaniform
sensilla, mostly on the forewings, whilst good fliers, like true flies, bees and wasps, may have 6-7 times as
many as the locust, arranged in 6 groups or so. The campaniform sensilla are proprioceptors, detecting
flexing and torque in the wing cuticle. Strains in the wing cause the domes to reversibly buckle, activating
the sensory dendrite underneath. They can thus detect changes in position of the wings. Their domes are
elliptical, rather than round, and they are aligned in such a way that they are most sensitive to wing twisting
(pronation and supination) such as when the wing changes its angle of attack through the wing stroke-cycle.
The main pattern of wing movement is generated by the central nervous system. Each segment of the
insect body has a pair of ganglia that are more-or-less fused into a single ganglion, situated ventrally and
connected to the ganglia in other segments by the double ventral nerve cord. The ganglia of some adjacent
segments may also be fused together, especially in the head and abdomen. These act as local processors
or 'brains'. The ganglia of the mesothoracic and metathoracic segments, the wing-bearing segments, are
chiefly responsible for controlling the wings. The brain is not directly required. Some of the anterior
abdominal ganglia may also contribute. These form a central-pattern generator that generates the
correct pattern of firing to move the wings even in the absence of sensory input. However, sensory input,
chiefly from the campaniform sensilla of the wings is essential to maintain the correct frequency of wingbeat
and also in the control fine movements of the wing, such as wing twisting.
The antennae are also involved in regulating flight speed. Both the strain acting on them by the moving air,
and the vibrations this causes, is sensed by Johnston's organ in the base of each antenna, giving a
measure of air speed. Initiation of flight can be brought about by loss of contact between the feet (tarsi) and
the ground, by pinching the abdomen, but this soon stops unless air is kept blowing on sensory bristles on
the insect's face. Thus, the head may not be needed to initiate flight, but it has a role in maintaining it and
in regulating speed.
The importamce of feedback from the sensory organs is illustrated by the observation that many insects
can continue to fly with one of their four wings missing. Dragonflies are especially noted for this, being able
to compensate and maintain stability in the air despite losing one of their hindwings, though their speed will
be reduced due to a loss of thrust.
The hindwings typically provide most of the thrust, each hindwing providing about 35% of the thrust in a
flying locust. The forewings may be toughened top provide protection for the hindwings, forming
parchment-like tegmina (singular tegmen), or in the case of beetle the very hard elytra (singular elytron)
or wing-covers. In many true bugs, Hemiptera, only the basal part of the forewing is thickened and these
are called hemelytra. In true flies, Diptera, the hindwings are reduced to the small club-like halteres,
which beat out of phase with the forewings and act as sensory gyroscopes to maintain flight-stability (they
are loaded with campaniform sensilla).
It is important that the forewings do not interfere with the hindwings during flight. In locusts, cocokroaches
and dragonflies the four wings are kept separate but each pair beats out of phase with the other. For
example in the locust the wings beat about 17 times per second and the forewings lead the hindwings by
about 6-8 millseconds. Some insects solve the problem by coupling the wings together. For example some
moths and butterflies have jugal coupling, in which a toothlike process extending from the hind-margin
(anal margin) of the forewing, called the jugum, fold under the hindwing in flight, so that forewings and
hindwings are locked together and function as a single pair of wings. This is much like cutting a slit in the
margin of a sheet of paper and slotting another sheet into it. In some moths, the mechanism used is
frenulo-retinacular coupling, in which a spinelike frenulum on the fore-margin (costal margin) of the
hindwing hooks under the loop-like retinaculum on the underside of the forewing. Amplexiform
coupling, seen in some butterflies, involves no special hooks, but the wings overlap significantly.
It is indeed very informative, from an engineering perspective amongst others, to compare insects with
vertebrates. Both are highly evolved and advanced animals. Insects should not be under-estimated!
Insects often have different and novel solutions to life's problems.
Climbing in Insects
the insect foot is a remarkable mechanical device. The five segments of the tarsus can bend to take the
insects weight, and the pretarsus has special equipment for gripping different surfaces. The pretarsus has
a pair of claws (pretarsal claws) and between these is typically a retractible cushion called the arolium.
The claws are used to grip a rough surface, whilst the arolium, aided by an oily secretion, is used to grip
smooth surfaces. This allows most insects to walk up walls and across ceilings, or up plant stems and
underneath leaves. Consider ants, they can support up to 100 times their body weight before their feet
detach!
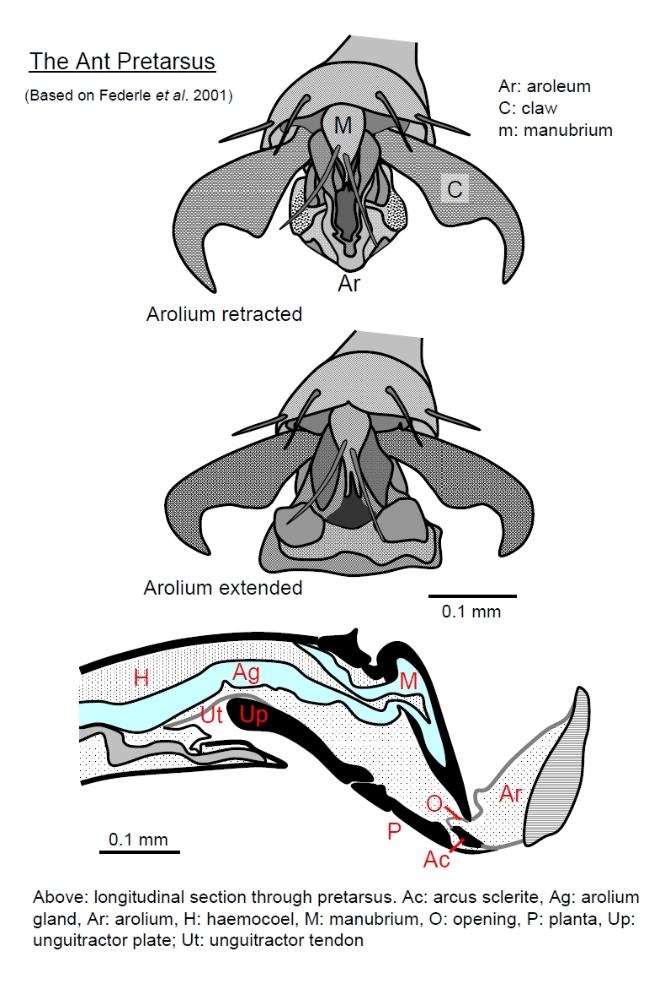
The mechanisms by which the arolium grips very smooth surfaces is not entirely understood. The
secretion doe snot seem to act like simple glue. Clearly intermolecular forces must be involved. The figure
below illustrates the anatomy of the ant pretarsus, externally in frontal view and internally in side-view:
vertebrates. Both are highly evolved and advanced animals. Insects should not be under-estimated!
Insects often have different and novel solutions to life's problems.
Climbing in Insects
the insect foot is a remarkable mechanical device. The five segments of the tarsus can bend to take the
insects weight, and the pretarsus has special equipment for gripping different surfaces. The pretarsus has
a pair of claws (pretarsal claws) and between these is typically a retractible cushion called the arolium.
The claws are used to grip a rough surface, whilst the arolium, aided by an oily secretion, is used to grip
smooth surfaces. This allows most insects to walk up walls and across ceilings, or up plant stems and
underneath leaves. Consider ants, they can support up to 100 times their body weight before their feet
detach!
The mechanisms by which the arolium grips very smooth surfaces is not entirely understood. The
secretion doe snot seem to act like simple glue. Clearly intermolecular forces must be involved. The figure
below illustrates the anatomy of the ant pretarsus, externally in frontal view and internally in side-view:
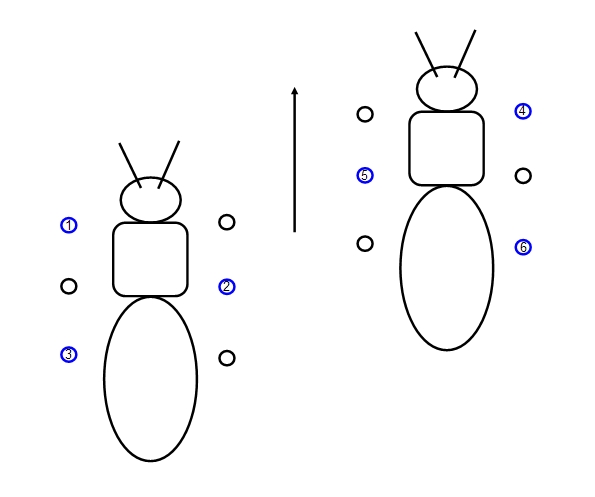
Above an insect taking a 'step' with the circles showing those feet in contact with the ground.
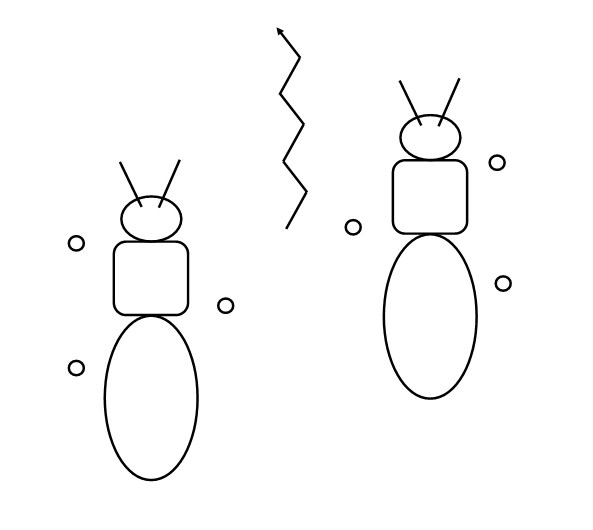
However, you may not be surprised that this is a simplification. Some insects show a different walking
pattern - they keep the tripod but lift the other three feet in sequence, one at a time, so that 4 or 5 feet are
in contact with the ground at any one time. This increases stability and also prevents pivoting so that the
insect can move in a straight line, as illustrated below:
pattern - they keep the tripod but lift the other three feet in sequence, one at a time, so that 4 or 5 feet are
in contact with the ground at any one time. This increases stability and also prevents pivoting so that the
insect can move in a straight line, as illustrated below:
An alternative walking pattern in some insects. The three numbered blue circles represent
those feet being lifted from the ground, the numbers indicating the order in which they are
raised.
those feet being lifted from the ground, the numbers indicating the order in which they are
raised.
Generally speaking, having fewer feet on the ground at any one time increases stability but reduces
speed. Thus some insects will switch their walking pattern, keeping fewer feet on the ground at higher
speeds. When they run, some insects have only two feet on the ground at any one time.
Crawling in Insects
Insect larvae may be capable of walking, but many crawl. In addition to the six thoracic legs, many insect
larvae can protrude pseudo-legs or prolegs from their abdomen. In the caterpillars of some moths and
butterflies a wave of muscular contraction travels from the rear to the front, with the prolegs and then the
thoracic legs advancing forward in turn, beginning with the rearmost, as the wave reaches them. The
haemolymph provides hydrostatic pressure, so that the larva is pressurised, and when muscles in the
body wall of a segment contract this pressure increases, protruding the prolegs by hydrostatic pressure.
(The prolegs are essentially inflatable). Specialised body wall muscles maintain the internal pressure.
Inchworm or looping movements may assist: arching of the caterpillar helps bring forward the prolegs as
the rear advances, and then straightening out of the body advances the front section bearing the
thoracic legs.
One of the characteristic features of insect muscles, especially those in larvae, is supercontraction. In
vertebrate muscles, each muscle fibre contains myofibrils, bundles of protein fibres: actin and myosin
filaments parallel to its long axis. Mobile, lever-like cross-bridges form between the myosin and
neighbouring actin filaments when the muscle contracts, pulling the filaments over one-another and
shortening the muscle. Along the length of each myofibril, the muscle is divided into sarcomeres, each
sarcomere containing an array of actin and myosin and separated from neighbouring sarcomeres by
z-bands (z-lines or z-discs), one z-band at each end of the sarcomere. The actin filaments are joined to
one z-band at one end, but the myosin filaments are not attached and occupy the centre of each
sarcomer. The actin (thin) and mysoin (thick) filaments slide past one-another as the muscle shortens.
The z-bands thus place a limit on how much the muscle can contract - once the myosin filaments hit the
z-discs as the sarcomere shortens, which happens when the sarcomere length equals the myosin
length,the muscle can contract no more. Vertebrate muscles can contract no more than 50%. In many
insect muscles, however, the z-bands are perforated, with pores through which the ends of the myosin
filaments can pass. Thus the sarcomere can shorten much more, to a length shorter than the length of
the myosin filaments. These muscles can shorten by 76%, a phenomenon called supercontraction. This
supercontraction partly explains the great flexibility of the bodies of many insect larvae, assisting their
locomotion.
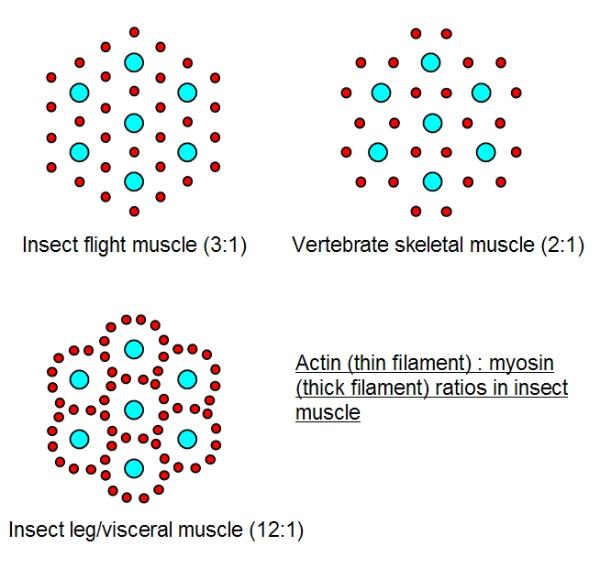
Another curious feature of insect muscles is that they have more actin filaments surrounding each myosin
filament. In vertebrate muscle, each myosin is surrounded by six actins and each actin by three myosins (
a 2:1 actin : myosin ratio). In most insect flight muscles each mysoin is also surrounded by six actins, but
each actin is adjacent to only two myosins (a 3:1 ratio). In some insect leg muscles there may be as many
as 12 actins around each myosin. Initially this was thought to increase contractile force (power and
strength) by enabling more cross-bridges to form. However, measurements show that insect muscle is
similar in strength to vertebrate muscle, per unit of cross-section, sometimes slightly less. To be certain
one would have to measure speed and power generation in addition to strength - these three parameters
are not equivalent. At the moment the reason for these differences between insect and vertebrate muscle
are not certain, but the greater actin : myosin ratios in insect muscles are correlated to greater overlap
between the actin and myosin filaments and it is thought that they are related to supercontraction.
speed. Thus some insects will switch their walking pattern, keeping fewer feet on the ground at higher
speeds. When they run, some insects have only two feet on the ground at any one time.
Crawling in Insects
Insect larvae may be capable of walking, but many crawl. In addition to the six thoracic legs, many insect
larvae can protrude pseudo-legs or prolegs from their abdomen. In the caterpillars of some moths and
butterflies a wave of muscular contraction travels from the rear to the front, with the prolegs and then the
thoracic legs advancing forward in turn, beginning with the rearmost, as the wave reaches them. The
haemolymph provides hydrostatic pressure, so that the larva is pressurised, and when muscles in the
body wall of a segment contract this pressure increases, protruding the prolegs by hydrostatic pressure.
(The prolegs are essentially inflatable). Specialised body wall muscles maintain the internal pressure.
Inchworm or looping movements may assist: arching of the caterpillar helps bring forward the prolegs as
the rear advances, and then straightening out of the body advances the front section bearing the
thoracic legs.
One of the characteristic features of insect muscles, especially those in larvae, is supercontraction. In
vertebrate muscles, each muscle fibre contains myofibrils, bundles of protein fibres: actin and myosin
filaments parallel to its long axis. Mobile, lever-like cross-bridges form between the myosin and
neighbouring actin filaments when the muscle contracts, pulling the filaments over one-another and
shortening the muscle. Along the length of each myofibril, the muscle is divided into sarcomeres, each
sarcomere containing an array of actin and myosin and separated from neighbouring sarcomeres by
z-bands (z-lines or z-discs), one z-band at each end of the sarcomere. The actin filaments are joined to
one z-band at one end, but the myosin filaments are not attached and occupy the centre of each
sarcomer. The actin (thin) and mysoin (thick) filaments slide past one-another as the muscle shortens.
The z-bands thus place a limit on how much the muscle can contract - once the myosin filaments hit the
z-discs as the sarcomere shortens, which happens when the sarcomere length equals the myosin
length,the muscle can contract no more. Vertebrate muscles can contract no more than 50%. In many
insect muscles, however, the z-bands are perforated, with pores through which the ends of the myosin
filaments can pass. Thus the sarcomere can shorten much more, to a length shorter than the length of
the myosin filaments. These muscles can shorten by 76%, a phenomenon called supercontraction. This
supercontraction partly explains the great flexibility of the bodies of many insect larvae, assisting their
locomotion.
Another curious feature of insect muscles is that they have more actin filaments surrounding each myosin
filament. In vertebrate muscle, each myosin is surrounded by six actins and each actin by three myosins (
a 2:1 actin : myosin ratio). In most insect flight muscles each mysoin is also surrounded by six actins, but
each actin is adjacent to only two myosins (a 3:1 ratio). In some insect leg muscles there may be as many
as 12 actins around each myosin. Initially this was thought to increase contractile force (power and
strength) by enabling more cross-bridges to form. However, measurements show that insect muscle is
similar in strength to vertebrate muscle, per unit of cross-section, sometimes slightly less. To be certain
one would have to measure speed and power generation in addition to strength - these three parameters
are not equivalent. At the moment the reason for these differences between insect and vertebrate muscle
are not certain, but the greater actin : myosin ratios in insect muscles are correlated to greater overlap
between the actin and myosin filaments and it is thought that they are related to supercontraction.
The pretarsus articulates with the 5th tarsomere (5th tarsus segment). It consists of several cuticular plates
(sclerites), the pair of pretarsal claws and arolium (Ar). The manubrium (M) is the topmost (dorsal) sclerite and
and externally the planta (P) behind which is the unguitractor plate (Up). The unguitractor plate is operated by
muscles via a tendon, the unguitractor tendon (Ut).
When the insect takes a step the following sequence of events occur:
1. The claws contact the surface and retract;
2. If the do not grip the surface (i.e. the surface is too smooth) then they retract fully and the arolium unfolds and
extends, exposing the manubrium;
3. The arolium contacts the surface and adheres, assisted by glandular secretion;
4. The claws extend as the arolium deflates and detaches and the foot lifts off the surface.
The whole sequence of claw retraction and arolium deployment is triggered by muscular contraction pulling on the
unguitractor tendon. The sequence of these events is as follows:
1. The unguitractor tendon is pulled, retracting the claws and pulling the whole pretarsus back into the 5th
tarsomere.
2. The pretarsus is hinged dorsally with the 5th tarsomere and rotates as it is pulled back, planting the pretarsus
on the surface.
3. The foot is fully rotated and further pulling on the unguitractor tendon now brings the unguitractor plate level to
the surface, which pushes the arcus and planta upwards. This causes the arcus to rotate around the manubrium
(about a horizontal axis) and the manubrium pushes down on the two side-arms of the U-shaped arcus. This
pushing on the arcus unfolds the arolium downwards.
4. The arolium rotates downwards and pressure between the pretarsus and the surface expands the elastic arcus
sideways. Thus the arcus converts a contact pressure into a sideways force which pushes the arolium out
sideways. In some species of ants the dominant mechanisms for extending the arolium is different, instead as the
pretarsus rotates fully about the 5th tarsomere, the pressure of the tarsomere pushing on the ventral surface of
the pretarsus at the joint elevates hydrostatic pressure and pressure in the glandular secretions that fill the
arolium. Thus, in this case hydraulic pressure expands the arolium sideways.
Insect larvae can also climb, but sometimes by very different mechanisms. The abdominal prolegs of caterpillars
may be equipped with microscopic hooks (proleg crotchets), or they may be modified into suckers. Some
caterpillars have a special escape mechanism: if disturbed they suddenly drop down from the leaf on which they
were feeding by a silk thread (life-line) secreted by spinnerets in the head. At least two mechanisms of climbing
back up this silk thread have been described. One mechanism involves the silk being wound-up between the third
pair of thoracic legs, strung between them in figures of eight. The legs on one side will gather slack in the thread
as the larva twists to one side, then pass the slackened thread onto the front leg of the opposite side. This foreleg
then passes the thread to the middle leg of the same side, which then passes it onto the hindleg. The pattern
repeats with the larva twisting to the alternate side - winding the silk about first one hindleg then the other.
A second method involves first taking up the slack in the thoracic legs as before, then flexing the ventral side to
arch the body (i.e. arching the body forwards), bringing up one of the abdominal prolegs to take the slackened
thread. The caterpillar then flexes dorsally, straightening out its body again, so that the abdomianl proleg pulls the
thread taught, propelling the caterpillar up the thread a short distance. This movement repeats until the climb is
completed. (Diagrams of these two methods can be found in Brackenbury 1996).
(sclerites), the pair of pretarsal claws and arolium (Ar). The manubrium (M) is the topmost (dorsal) sclerite and
and externally the planta (P) behind which is the unguitractor plate (Up). The unguitractor plate is operated by
muscles via a tendon, the unguitractor tendon (Ut).
When the insect takes a step the following sequence of events occur:
1. The claws contact the surface and retract;
2. If the do not grip the surface (i.e. the surface is too smooth) then they retract fully and the arolium unfolds and
extends, exposing the manubrium;
3. The arolium contacts the surface and adheres, assisted by glandular secretion;
4. The claws extend as the arolium deflates and detaches and the foot lifts off the surface.
The whole sequence of claw retraction and arolium deployment is triggered by muscular contraction pulling on the
unguitractor tendon. The sequence of these events is as follows:
1. The unguitractor tendon is pulled, retracting the claws and pulling the whole pretarsus back into the 5th
tarsomere.
2. The pretarsus is hinged dorsally with the 5th tarsomere and rotates as it is pulled back, planting the pretarsus
on the surface.
3. The foot is fully rotated and further pulling on the unguitractor tendon now brings the unguitractor plate level to
the surface, which pushes the arcus and planta upwards. This causes the arcus to rotate around the manubrium
(about a horizontal axis) and the manubrium pushes down on the two side-arms of the U-shaped arcus. This
pushing on the arcus unfolds the arolium downwards.
4. The arolium rotates downwards and pressure between the pretarsus and the surface expands the elastic arcus
sideways. Thus the arcus converts a contact pressure into a sideways force which pushes the arolium out
sideways. In some species of ants the dominant mechanisms for extending the arolium is different, instead as the
pretarsus rotates fully about the 5th tarsomere, the pressure of the tarsomere pushing on the ventral surface of
the pretarsus at the joint elevates hydrostatic pressure and pressure in the glandular secretions that fill the
arolium. Thus, in this case hydraulic pressure expands the arolium sideways.
Insect larvae can also climb, but sometimes by very different mechanisms. The abdominal prolegs of caterpillars
may be equipped with microscopic hooks (proleg crotchets), or they may be modified into suckers. Some
caterpillars have a special escape mechanism: if disturbed they suddenly drop down from the leaf on which they
were feeding by a silk thread (life-line) secreted by spinnerets in the head. At least two mechanisms of climbing
back up this silk thread have been described. One mechanism involves the silk being wound-up between the third
pair of thoracic legs, strung between them in figures of eight. The legs on one side will gather slack in the thread
as the larva twists to one side, then pass the slackened thread onto the front leg of the opposite side. This foreleg
then passes the thread to the middle leg of the same side, which then passes it onto the hindleg. The pattern
repeats with the larva twisting to the alternate side - winding the silk about first one hindleg then the other.
A second method involves first taking up the slack in the thoracic legs as before, then flexing the ventral side to
arch the body (i.e. arching the body forwards), bringing up one of the abdominal prolegs to take the slackened
thread. The caterpillar then flexes dorsally, straightening out its body again, so that the abdomianl proleg pulls the
thread taught, propelling the caterpillar up the thread a short distance. This movement repeats until the climb is
completed. (Diagrams of these two methods can be found in Brackenbury 1996).
| Did you know? Many insects migrate great distances. For example, the small mottled willow moth (Spodoptera exigua) may migrate over 3000 km across the sea from Morocco to England, arriving mostly between August and October. The Monarch butterfly of North America may travel 3000 km north in summer, from its warmer overwintering grounds down south (in areas such as California). They often overwinter in the same region, even the same tree, year after year and fly about 5 m from the ground, but will soar to clear buildings, trees and mountain passes. |