| Building
Bodies from Slime: Plasmodia |
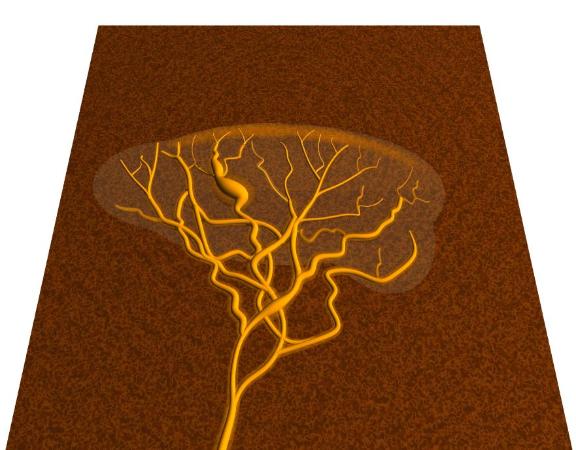
AQ Pov-Ray model of a slime-mould plasmodium.
Slime
moulds fall into two basic types: cellular, for example Dictyostelium, and plasmodial, e.g.
Physarum, which is modeled
above. Both are extraordinary classes of organisms! The cellular
slime moulds are amoebae which can come together, when needs
must, to form a basic multicellular organism! This makes them of
great scientific interest in studying multicellularity and the
evolution of multicellular life-forms and cell communication.
Plasmodial slime-moulds are extraordinary for being amongst the
largest living 'cells'. The plasmodial slime-moulds are also
known as myxomycetes and are the subject of
this article.
The
Plasmodium
The
pictures above show a computer Pov-Ray model of a slime-mould plasmodium. These creatures form
a sheet of mobile slime, only a couple of millimetres thick at
most but up to one metre in more diameter. The remarkable things
is, that despite their large size, these creatures are single
cells! They resemble single giant animal cells, but are
protoctistans, as is the single-celled amoeba which they resemble.
Amoebae are normally one millimetre across at most, but the
plasmodium slime-mould is in essence a giant amoeba. The
plasmodium actually begins its life as a tiny amoeba, but grows
and grows! Like an animal
cell,
the plasmodium consists of cytoplasm enclosed by a 'skin' called
the plasma-membrane. Whereas a typical
animal cell contains one nucleus, in contrast, a plasmodium
contains many thousands of nuclei (as required to maintain its
large size). The plasmodium is able to slowly crawl along the
surface, at about one centimetre per hour, in which case it
assumes a fan-shape, with the broad margin leading as the front
end, though it can easily change shape and start moving in
another direction. In essence, plasmodial slime-moulds are
giant, terrestrial and multinucleate amoebae. Note that although
a plasmodium is essentially a multinucleate cell, many
biologists do not consider them 'cells' on the basis that a cell
has a single nucleus (although many classical cells normally
have multiple nuclei anyway, such as muscle fibres and
osteoclasts). A better definition of cell is as a single
protoplasmic unit, bounded by a cell membrane, in which case
plasmodia are indeed multinucleate cells.
To facilitate this movement, the plasmodium forms a network of
vessels to transport its cytoplasm from one end to the other.
Plasmodia vary in colour, depending upon species, though most
are either transparent or bright yellow or white, some are
reddish, green or even blue in colour. Although they occur in
most habitats, they are most likely encountered crawling in the
leaf litter and inside rotting logs in damp woods. They feed by
smothering their food and absorbing it directly (a process
called phagocytosis). They engulf
bacteria, fungi, and decaying organic matter and can home-in on
potential food by responding to its odour. I have heard stories
of people keeping small plasmodia in petri dishes, and
entertaining people by placing food atone end of the dish, and
then watching the slim slowly crawl over to eat the food!
I once found two translucent plasmodia (almost totally
transparent) crawling across a felled log in a damp Devonshire
woodland. Each was about the size of a human hand. Pressing one
of the veins, it was surprisingly rigid, but squeezing it showed
the cytoplasm inside squirt along the vein in either direction
from the point of pressure. Cytoplasm ordinarily moves back and
forth along these veins in oscillations lasting about one
minute, with cytoplasm streaming along at up to 1.3 millimetres
per second. Superimposed on these oscillatory movements is a
general net movement of cytoplasm toward the leading edge.
The diagram below shows the structure of a small plasmodial
slime-mould.
Above: the structure of a plasmodial slime mould (redrawn from Fleischer and Wohlfarth-Bottermann, 1975). CFC, circular fibrils in cross-section; CFL, circular fibrils in longitudinal section; ECC, ectoplasm in cross-section; ECL, ectoplasm in longitudinal section; ENC, endoplasm in cross-section; ENL, endoplasm in longitudinal section; PI, plasma-membrane invagination;PL, plasma-membrane (plasmalemma) and PS, pseudopod.
First,
it should be noted, that in a plasmodium of at least moderate
size, the network of vessels are much more complex than depicted
either in the computer model or in the diagram above - many
finer vessels branch and ramify to form a lattice-like network,
rather like the veins in a leaf, especially toward the leading
edge where the finer veins are denser.
Notice that each vein consists of two concentric cylinders
inside the plasma-membrane sheath (PL) - the outer ectoplasm and the inner endoplasm. The ectoplasm is
stiff and gelatinous and contains longitudinal and circular
fibrils (and also radial fibrils which are not shown) which are
composed principally of rods of a protein called actin. Actin is a component
of the cell skeleton and is capable of exerting tension and
ordering water in the cytoplasm to form a stiff gel, such as the
ectoplasm. The endoplasm is free of these stiffening actin rods
and much more watery. The actin fibrils in the ectoplasm
contract in sequence, squeezing the fluid endoplasm along the
veins, rather like squeezing toothpaste along a tube. This
transports cytoplasm from the rear of the creature to the
advancing edge. When the time comes, ectoplasm can be mobilised
by simply dissolving the actin cytoskeleton and turning the
ectoplasm into endoplasm. Actin has the remarkable property of
being able to assemble rods (columns or struts) or dissolving
into fluid, as and when required! The pseudopods (literally
'false feet') absorb any food on the substrate as the slime
crawls along and are also assembled and disassembled as
required.
Plasmodia have some remarkable properties. If you cut one into
several smaller pieces, no matter, each fragment will continue
to move and crawl as an individual organism, but if it
encounters another piece of its former self, then the two will
fuse back together again! If, however, a foreign plasmodium
(differing genetically) is encountered, then the bigger will
usually devour the smaller!
Finally, when the plasmodium has fed sufficiently, it will crawl
out from hiding and ascend a tall structure (if one is
available) stop moving and then transform into a spore-producing
structure.
This is when plasmodia are most often seen - as foam-like
material on grass or the trunks of trees. In this non-motile
state, the fan-like shape with its veins disappears and the
whole resembles a foamy mass which easily fragments on touch,
rather like shaving foam. (It is also cool to the touch and has
a subtle slightly minty odour in my opinion, an odour which is
unmistakable). I once saw a specimen about 30 cm long and 5 cm
wide streaked vertically along the outside of an old oak trunk,
about 2 metres from the ground. This state is modelled in the
computer graphics below.
Above: the plasmodium is preparing to spore and transforming into a sporing mass called an aethelium. This is the form that most people see slime moulds in, and they usually mistake them for foam that some one has sprayed! This is the type of structure formed by the large Fuligo and Physarum polycephalum species. Actually, many species do not form these structures, but instead form one or more rounded nodules or clusters of tiny stalked sporangia, very similar to those produced by cellular slime moulds but usually occurring in groups rather than singly. These stalked structures are easily seen on rotting logs, but are missed by most people because of their small size - they are slimy and usually brightly coloured and much smaller than toadstools. An example of the stalkless (sessile) type that nevertheless has distinct capsules (sporangia) is shown below. The stalkless types are often larger than the stalked sporangia, with each capsule often several centimetres across in some species.
A cluster of stalkless slime mould sporangia.
Eventually,
these structures, either the foam-like mass or discrete
sporangia capsules, will harden and turn darker and eventually
the whole organism turns into a mass of dark powder enclosed by
a dry skin. This 'skin' cracks and the spores escape, to be
carried away by wind or rain. This powder is a mass of spores have a characteristic
minty odour (never sniff spores of any slime mould or fungus if
you are asthmatic!). If they find a suitable place in rich soil,
then the spores will germinate, releasing a single microscopic
amoeboid cell, to continue the cycle over again.
The advantage of this complex life-cycle is that it better
enables the creature to find food when food is scarce, since the
large plasmodium can cover more ground than a microscopic
amoeba, and it also enable the creatures to crawl high up tree
trunks and the such, to better disperse their spore over long
distances.
Overview
of the life-cycle
The
sporing bodies become brittle and dry structures which
disintegrate to release spores. The parent plasmodium is diploid (possessing two sets
of chromosomes, 2n) and spores are produced by meiosis (a reduction division)
and so each is haploid (possessing only one
set of chromosomes, n). Each spore is a single haploid cell,
with a single nucleus, encased in a resistant spore-coat or
'shell' (test). The spores are easily spread by wind, rain,
animals and vibrations. In suitable conditions, the spores
germinate - the tough spore coats break-open and the single
amoeboid cell escapes. When immersed in water each amoeba can
develop two flagella used for swimming, with one tinsel
flagellum leading and one smooth flagellum trailing. If
conditions dry again then the cell loses its flagella and
returns to amoeboid crawling. If conditions become unfavourable
then each amoeba can encyst by secreting a protective shell
around itself and becoming dormant; 'hatching' again when
conditions become suitable.
If two compatible amoebae meet then they may fuse together, in a
sexual process, forming a single diploid amoeba or zygote.
Compatible haploid amoebae may derive from spores produced from
the same parent plasmodial (homothallic strains) or they have to
be derived from different parents, ensuring cross-fertilisation
(heterothallic strains). This diploid amoeba grows as it feeds
on bacteria and other micro-organisms, eventually becoming a
plasmodium by repeated nuclear mitosis / division (though
plasmodia of the same genetic type will fuse and this possibly
contributes to plasmodial growth). Interestingly the division of
the many diploid nuclei, of which there may be millions, is
synchronised. In some species each haploid spore gives rise to a
haploid plasmodium and two compatible plasmodia mate by fusing
into a diploid plasmodium. If starved then the plasmodium may
develop into a dormant sclerotium: consisting of a mass of
dormant multinucleate spherules. If l;ight is also present then
it may develop into a mass of fruiting bodies and sporulate.
When the plasmodium is preparing to sporulate and has found a
suitable elevated position, then it loses the vein-like
structure and develops bumps or papillae over its surface. Each
papilla may elongate into a stalk (inside which granules deposit
to give the stalk strength) by drawing up slime from the
surrounding plasmodium, and is capped by a tiny spherical,
discoid or elongated sporangium containing the spores
and capillitial
threads.
These threads aid spore dispersal by twisting about as they dry.
Other species simply transform into one or a group of often
large stalkless sporangia, or else the plasmodium transforms
into a foam-like mass (aethelium) which directly
transforms into a dry powdery mass of spores, as in Fuligo. The surface of the
aethelium is covered in blebs and the aethelium is quite thick
as protoplasm is drawn up into a mound. Some species form a plasmodiocarp which keeps the form
of the original plasmodium, with its netlike veins, but is
immotile like all the sporing forms. In some forms many
sporangia may fuse together into a compound structure which is
sometimes known as a pseudoaethelium when it resembles an
aethelium. Generally, the sporing structure, regardless of type,
is covered in a thin 'skin' or peridium which dries and breaks
apart to release the spores.
Haploid amoebae may develop a flagellum and develop into a
flagellated stage, which may also transform back into a
non-flagellated amoeba. These flagellated forms may also
germinate directly from a spore and may also fuse to form a
non-flagellated diploid zygote. In short, the haploid amoebae
can alternate freely between flagellated and non-flagellated
forms, according no doubt to certain stimuli. Like many amoebae,
they may also encyst, surrounding themselves in resistant shells
and entering a dormant period and then emerging from the cyst
again when conditions are suitable.
Field
Observations
Is
it any wonder that for a long time biologists could not decide
what to classify plasmodial slime-moulds as - sometimes they
were included with the fungi, sometimes with animals and finally
(or not?) with the protoctistans - which includes all sorts of
creatures that do not neatly fit anywhere else!
Plasmodia are food for some creatures - certain insects, like
some fly species, make a living out of laying eggs in plasmodia,
the maggots then eat part or all of the plasmodium before
pupating. I once saw a batch of maggots eat part of a large
plasmodium that had settled down to spore (the same one on the
oak tree mentioned above) before pupating as strange spiny pupae
which emerged into what I never saw. They did not destroy the
whole slime mold, however, which went on to produce millions of
spores! I believe that a certain species is eaten in Mexico as a
delicacy (?) but they are generally not edible. Years in which
slime molds grow especially well have been known to create
public scares as people report seeing strange pulsating blobs, mistaken for aliens
from outer space!!
Above: a plasmodium (transforming into an aethelium) about one foot in length has formed a foam-like streak on the bark of this old oak tree (the tree is estimated to be about 400 years in age) - much of its is covered by the ivy. [Unfortunately, this is an old photo from an old camera that wouldn't let me get any closer without losing focus.] This plasmodium is stationary, having left the fan-like crawling stage (the plasmodium proper) and begun to transform into a sporing stage or aethelium. This is probably a species of Fuligo (probably Fuligo septica). Over the next few days the mass dried, and beneath the hardened crust was a mass of powder with a minty odour (do not sniff spores if you suffer from asthma!). Some plasmodia can dry reversibly into a dormant stage (sclerotium) that comes back to life on application of water, but I think this one was in the terminal fruiting stage. Slime moulds have specialised predators, and some sort of fly laid its eggs in this one, the hatchling maggots devoured part of the slime before pupating, several days later, as spiny pupal cases from which adult flies emerged (I never got to see the adults). However, most of the slime mass remained, so this creature did its job in producing plenty of spores. They are very hard to destroy by mechanical means - no matter in to how many parts they are cut, broken or crushed, the parts go on living and can rejoin if the plasmodium is still in its motile stage!
Above: a cross-section through a plasmodial strand (vein). Bundles of actin filaments in the outer (gelatinous) attached to the plasma membrane at membrane invaginations (clefts) contract (probably with the aid of mysosin) ectoplasm contract, narrowing the vein and squeezing the endoplasm along it. Endoplasm may move at speeds of 1 mm/s in this fashion. Actin and myosin form bundles called microfilaments or microfibrils. In animal muscle these bundles (called myofilaments) bring about contraction of the muscle.
More field observations:
This
mass of spores is all that remains of a sporing mass probably
belonging to Reticularia
lycoperdon (= Enteridium
lycoperdon). A few days earlier,
the plasmosdium had turned into a white hemi-spherical mass
(about the size of a fist in this case) attached to this fallen
log (the white 'skin' or peridium enclosing the developing mass
of spores). This browns as it ripens and turns into a mass of
spores. Unfortunately, when I returned a few days later the
impressive sporing body had already disintegrated! The slightest
tap to the log would send a cloud of spores shooting into the
air. The moral of the tale is - when walking in the countryside
always carry a camera, because you never know what you may find!
This type of sporing body, in which the plasmodium rounds up to
some extent and then forms one or a few large sporing masses is
called an aethalium. The aethalium may be encased in a
common toughened layer, called the cortex, as it was in
this case.
This is a species I have seen on several occasions, including a pinkish-white aethalium about 3 cm long, that crawled out of woodwork overnight in a bathroom, though again I failed to get photos of it or take samples for microscopical analysis, but as Bruce Ing in his book 'The Myxomycetes of Britain and Ireland - An identification handbook (New enlarged edition)' points out, this species is often found on woodwork indoors.
Trichia
After consulting Bruce Ing's handbook and other sources, I have determined this specimen as Trichia botrytis (var. botrytis) with its pyriform (pear-shaped) sporangia which vary considerably in color (though this depends in part on how authors separate out related taxa) but typically purplish-brown to reddish to almost black. Characteristic are the dark stalks (dark yellow to brownish). Trichia flavicoma is similar but occurs on leaf litter, whereas Trichia botrytis occurs on fallen branches and trunks, as seen here (especially those of oaks and conifers). In many slime molds, such as this, the plasmodium transforms into a mass of small sporangia, which are often born on short stalks (as in this case). The whole mass (of which half is visible here) covered about two hand-spans of this rotting ash log. The sporangia at first appear pink on short white stalks (can you see one that is still pink?) but by the next day they mature to black. This entire mass could be from a single plasmodium.
Above and below: Trichia botrytis sporulating on a rotting ash log. Photos courtesy of Chris Pearce (copyright). The plasmodium has formed a mass of discrete sporangia, each sporangium with its own stalk.
Sporangia
vary considerably in colour and shape between different species
and are a useful aid to identification of the species. Each
sporangium is covered in a layer called the peridium, which gives the
structure a shiny appearance in this case. The stalk typically
continues some distance into the spore capsule as an extension
called the columella to which a network of elastic fibres,
called the capillitium, may be attached. When
the capsule opens, these fibres expand, helping to disperse the
spores. In other cases the capillitium is replaced by separate
ropelike filaments wit spiral thickenings which twist and turn
upon drying, scattering the spores. The capsule opens upon
drying, when mature, to release the spores. Depending on species
it may split apart in an irregular fashion, or along
predetermined lines, or a lid may detach. The basal part of the
peridium may remain as a cup holding the mass of dispersing
spores, such a cup is called a calyculus.
Lycogala
Above: Lycogala, probably Lycogala terrestre, which forms small, spherical, puffball-like sporing bodies (aethalia) pink or orange, developing from an orange, peach or cream-coloured plasmodium. Becomes pale or grey at maturity and breaks down into a powdery pink spore mass. Each sporing body is about 0.5 to 1.5 cm in diameter.Note the pale scales on the outer cortex and the pink/peach/salmon-pink spore mass (which fades to ochre / rust-colored). The aethalia are pulvinate (cushion-like) and pale buff (yellow-brown) or pink and found on or around dead or fallen wood.
Observations on Plasmodium Locomotionand Sporulation in Fuligo
This plasmodium emerged during the course of a day in late August from a bed of bark chips on to a wall (the highest point). The picture above was taken at 16:29. The same plasmodium was photographed again at 18:52, almost 2.5 hours later. It had advanced 7 to 8 cm in this time, a speed of about 0.5 mm per minute. The cytoplasm inside the veins of a plasmodium can move 1.3 mm / min, but net locomotion is slower as the tubes periodically reverse flow and pump cytoplasm backwards at regular intervals. More of the plasmodium was emerging from the bark chips, confirming that the entire organism was about 21 inches (about 50 cm) long, about 8 inches of which are shown below. The main body was now about 10 inches long, the rest was a trail consisting of a network of protoplasmic veins which dwindled as the plasmodium advanced. It was evident that the whole mass was gathering itself on the wall as a dense mass, probably for sporulation.
Above: a fragment of the plasmodium migrating under the microscope.
The fan-shaped leading edge of advancing pseudopods can be clearly seen above.
Click
images for full size.
I guessed correctly that the plasmodium was coming towards the
end of its journey as the front slowed down and began
accumulating more mass as the rear caught up with it.
A diminishing network of slimy veins trailed another 10 inches (25 cm) or so behind the main body of the plasmodium, winding their way through the wood chips, betraying the path taken. As their substance was being transported to the leading edge of the plasmodium these tubes narrowed and eventually retracted altogether to join the main body.
The
next day: having adopted its final elevated position the rear
was brought up and the whole plasmodium formed a somewhat
smoothened and compacted mass which is drying into an aethalium
(a large fruiting or sporing body). The outer crust (cortex)
is already dry, with a chalky texture and easily disintegrates
with the lightest touch (left). Indeed, the cortex was limy. The
inner material was still somewhat spongy.
Within 2 days the aethalium had largely disintegrated into a
mass of spores (below). Each spore, if it finds suitable
conditions, could germinate to form a new microscopic amoeba and
begin the cycle again! Under the microscope, this brown mass was
seen to consist entirely of spores (along with a small amount of
bacterial contamination).
Above: some of the spores as seen under the microscope. The fate of these spores depends on the species. Generally, the spore, which is diploid, undergoes meiosis before germinating. At germination, the spore wall either forms a pore or cracks open and 4 haploid amoeboflagellate cells emerge. Some of these cells may be flagellated, whilst the others are amoeboid. Amoeboid cells (myxamoebae) are suited to crawling in drier conditions, whilst flagellated cells (swarmer cells) are suited to swimming in water. Those amoebae that find themselves in plenty of water can produce flagella, and likewise flagellated cells can lose their flagella and become amoeboid. In apomictic forms, or forms capable of switching to apomixis, there is no meiosis and a diploid cell emerges from the spore instead of 4 haploid ones. Myxomycete spores are typically between 5 and 15 micrometres in diameter (one micrometre = one thousandth of a millimeter). Note that there are no capillitial threads in this species. However, a more thorough examination managed to find capillitial fibres, so they are present but scarce.
Above: a capillitial fibre surrounded by spores. The fibre is hyaline (translucent) and not comprised of lime. However, small masses of non-crsytalline lime are intermingled with the spores, as shown below:
White
calcareous nodes in the capillitum are diagnostic of var. septica,
as is the pale whitish-yellow cortex. This variety is, as noted
by Bruce Ing in his handbook, often found on tan bark, as here
(and also sawdust and rotting wood). In the British isles, it is
more common in the warmer parts, particularly the southeast of
England, as here.
The
fact that the fruiting body is an aethalium and that the spore
mass is dark brown and the presence of lime all suggest that
this myxomycete is a member of the order Physarales. Using
Stephenson and Stempen's key (in: Stephenson, S.L and H.
Stempen, 1994. Myxomycetes, A handbook of slime molds, Timber
Press) we may now be able to determine the species. A limy
cortex narrows us down to Mucilago
crustacea
or Fuligo
septica
as likely candidates. The former has crystals of lime in the
cortex, the latter has granules of lime. Ours lacks crystals and
has irregular granules, suggesting that it is in fact Fuligo septica which also has hyaline
capillitial fibres, sometimes sparse (and joined by nodes of
lime). The aethalium of this species is often large and its
habitat consists of decaying wood and bark, forest leaf litter,
etc. We have thus come to the decision that this is Fuligo, and probably Fuligo septica.
Meanwhile, the isolated fragment, kept out of direct sunlight and in damper conditions chose a different developmental fate: it migrated only a short distance before appearing to transform into a fourth type of structure: a plasmodiocarp, which retains the form of the veins of the original plasmodium to some extent, although it seems to have gone some way towards producing what look like irregular sporangia. Since sporing body type is a main diagnostic feature used in keys, it is unlikely, however, that the same plasmodium could be capable of producing a plasmodiocarp in addition to the aethelium. Slime moulds can also form a mass of hardened cell-like units under unfavourable conditions, such as drying out or low temperatures, such a mass being called a sclerotium and I wonder if that is what has happened here. I shall carry on observing and see what happens. A closer examination should settle the issue.
This
transformation corresponded to the addition of moisture and
corresponds to an interesting observation, again noted in Bruce
Ing's handbook, that in the presence of moisture the plasmodium
of this variety tend to transform into a 'brain-like mass' of
plasmodiocarps without the cortex of the aethalium.
Meanwhile, back outside ... another plasmodium of the same species and a similar size has crawled out of wood chips onto the opposite wall! I was too busy in the day to catch its migration, but here it is as a compacted mass getting ready to turn into an aethalium.
The appearance of the plasmodium, the lack of capillitial fibres, the production of an aethalium and the limy nature of the cortex all suggest that this myxomycete is either Mucilago crustacea or Fuligo septica; microscopic examination supports the latter. The coloration of Fuligo septica is quite variable, but the range includes the colours we see here.
This outer layer of tissue enclosing the mass of developing spores is called the cortex. Some regard the aethelium as a mass of fused sporangia, though there is no evidence of that in the shape and texture of it in this case.
Intelligent
Slime
Plasmodia
are surprisingly intelligent, in an alien kind of way.
experiments have shown that plasmodia will distribute themselves
between distributed discrete food sources, depositing a feeding
mass at each food location whilst the rest of the plasmodium
reduces to a series of connecting tubes, or continues searching.
In this way they will gradually assume the most efficient form,
in which the connecting tubes take the shortest paths for
efficient transport. For example, experiments have shown that
placing food strategically to minimise the locations of urban
settlements in a model landscape, the connecting tubes will
exhibit the most efficient transport network, matching for
example the train lines in Tokyo, or the motorways in Britain,
with occasional discrepancies where human engineers and
architects never chose the most efficient route for various
reasons. Similarly, if food is dispersed at each end of a maze,
the exploring plasmodium will connect masses of protoplasm at
each feeding station with connecting tubes following the
shortest route through the maze. These problems are
mathematically complex and hard to solve, but the plasmodium
solves them using basic rules laid down in its genetically
determined behavioural patterns.
Plasmodia also have some form of memory. They will learn to
anticipate a regular change in the environment, such as a cold
snap which slows them down and change their behaviour at the
correct point in time even when the cold snaps are withheld
after a period of training.
How does one part of the plasmodium signal to another part?
Clearly, plasmodia are frequently too large for chemical signals
to spread by diffusion (this would simply take far too long).
Either chemical signals are transported in the streaming
protoplasm (again probably too slow to explain some of the
behaviours of plasmodia) or the signal is electrical or
mechanical. Clearly more research is needed to elucidate the
mechanisms of cellular intelligence in slime
moulds!
Slime
Mould Habitats
Slime
moulds are generally associated with cool and moist habitats,
such as rotting wood or leaf-litter. Some are quite generalist,
others have a preference for either the bark or leaf-litter of
coniferous or deciduous trees (coniferous bark is generally more
acidic) and some prefer surfaces covered with mosses or
liverworts (bryophytes). Some specialise in
devouring the numerous bacteria and/or fungi found within and on
animal dung. So-called 'snowbank' or
'snowmelt' myxomycetes are found sporing at the edges of thawing
snow in spring and early summer in the Alps of Europe and in
North America. Perhaps surprisingly, others occur in more
extreme environments, such as Arctic tundra and even deserts.
Desert species are active for just a short time following rain
and thrive upon, for example, the remains of rotting cacti.
Above: Fuligo septica getting ready to sporulate. This slime mold occurs in woodlands and was found here on a moss-covered tree stump.The bright yellow color of the aethalium cortex suggests that this is var. flava (which also has yellow rather than white capillitial nodes). this variety is found on rotten wood, sawdust, forest litter and mosses above buried wood and resembles 'scrambled egg spilt on a log'. Notice the white hypothallus around the margin. the plasmodium of this form is yellow.
More Slime Moulds
Above and below: Ceratiomyxa fruticulosa. The plasmodium (a phaneroplasmodium) is translucent, watery and white or pale yellow. The beautiful sporangia looked almost like tiny ice crystals.
Below: This is probably the yellow form of Ceratiomyxa fruticulosa. The plasmodium can be still be seen in places, as the sporing bodies develop, clinging to the bark of this fallen birch branch, which was covered in patches of the slime mold from one end to the other. Although there is some branching of the sporing bodies, this is probably the fruticulosa variety. The var arbuscula of this species forms distinctly tree-like structures.
Below: Trichia
The white form above could be Trichia pessimilis: immature sporangia are often white and darken to yellow-brown when mature. To determine the species precisely requires microscopical examination of the spores and elaters, though the form of the sporangia narrows it down to a few species. Microscopical examination of a tiny sample (I never sample more than necessary) of the above specimen revealed immature spores and no elaters and determination was not made with this immature material. The plasmodium is white. It is found on rotten logs without mosses, but not generally logs that are at an advanced soft stage of decay.
Above: Trichia erecta, T. botrytis, T. flavicoma, T. munda, and T. decipiens regularly form simple stalked sporangia. These species can be distinguished to some extent by color and sporangium morphology if the sporangia are mature (I would say the ones above are not fully mature) and by spore color and spore and elater morphology as seen under a microscope. (No samples were taken in this case for microscopical study). T. erecta sporangia are generally less well clustered. T. flavicoma occurs on leaf litter and vegetable compost so can probably be ruled out here. T. munda forms scattered sporangia on mosses on bark of living trees or occasionally on mossy fallen branches and so can also be ruled out. T. botrytis often forms sporangia clustered on united stalks and the sporangia have a mottled texture. By a process of elimination, this leaves T. decipiens as the most likely species here.
Useful Textbooks
Stephenson, S.L. and Stempen, H. 1994. Myxomycetes: a handbook of slime molds. Timber Press, Inc. USA.
Ing, B. 2020. The myxomycetes of Britain and ireland: an identification handbook. (New enlarged edition). The Richmond publishing Co. Ltd. Great Britain.
| Article
updated: 4 July 2015 11 Aug 2016 28 Aug 2017 06 Sep 2017 11 Jun 2018 19 Oct 2020 16 Dec 2020 |